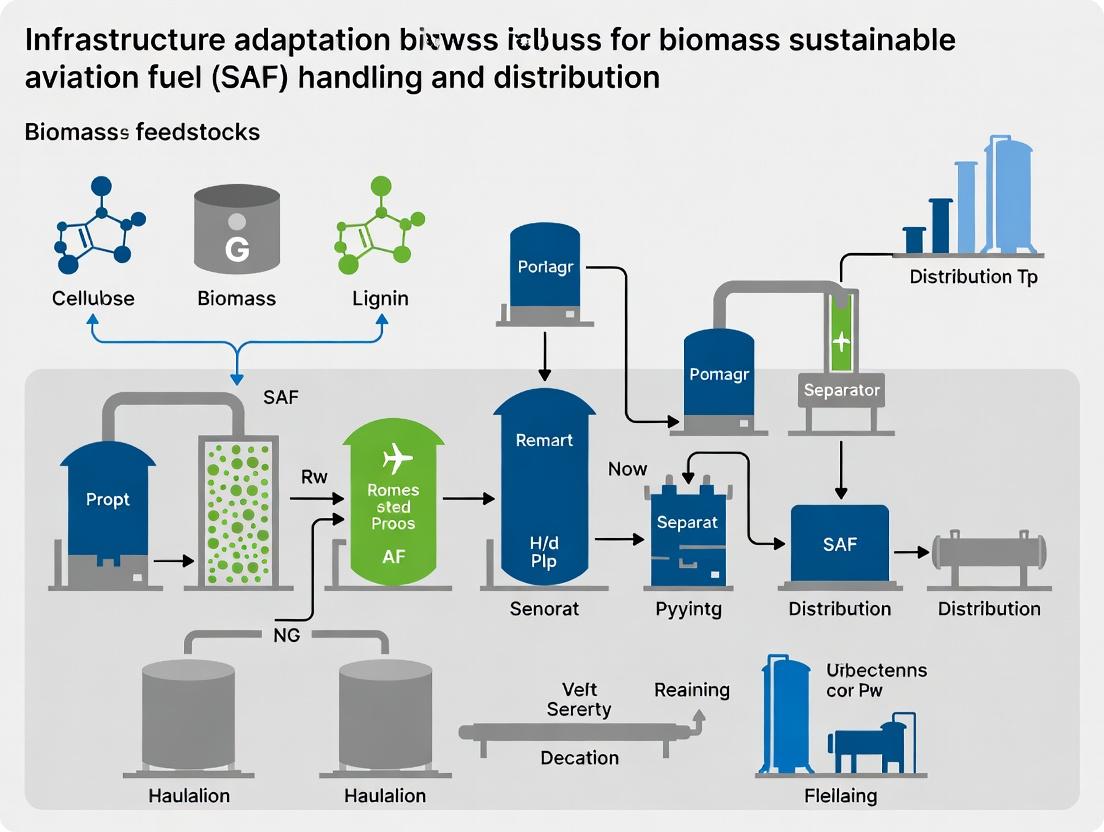
Biomass to Wings: Adapting Infrastructure for the Scale-Up and Distribution of Sustainable Aviation Fuel (SAF)
This article provides a comprehensive analysis of the infrastructure challenges and adaptation strategies required for the large-scale handling and distribution of biomass-derived Sustainable Aviation Fuel (SAF).
Biomass to Wings: Adapting Infrastructure for the Scale-Up and Distribution of Sustainable Aviation Fuel (SAF)
Abstract
This article provides a comprehensive analysis of the infrastructure challenges and adaptation strategies required for the large-scale handling and distribution of biomass-derived Sustainable Aviation Fuel (SAF). Targeting researchers and process development professionals, it explores the fundamental properties of biomass SAF that necessitate infrastructure changes, details current methodological approaches for material compatibility and logistics, addresses key troubleshooting and optimization hurdles for cold flow and contamination, and validates strategies through comparative analysis with conventional and other alternative fuels. The synthesis offers a critical roadmap for integrating SAF into the global aviation energy ecosystem, highlighting implications for supply chain design and regulatory frameworks.
Understanding Biomass SAF: Key Properties Demanding Infrastructure Evolution
Technical Support Center: Troubleshooting Guides & FAQs
FAQ 1: Why is my HEFA-SAF yield lower than expected when using used cooking oil (UCO) feedstock?
- Answer: Low yields in Hydroprocessed Esters and Fatty Acids (HEFA) pathways using UCO are commonly due to high free fatty acid (FFA) content and impurities. FFAs can cause catalyst poisoning (e.g., hydrotreating catalysts like NiMo/Al₂O₃) and soap formation during pre-treatment, reducing hydrocarbon conversion efficiency.
- Troubleshooting Protocol:
- Feedstock Analysis: Quantify FFA content via titration (ASTM D664). If >2%, pre-treatment is required.
- Pre-Treatment Step: Implement an acid esterification pre-treatment. React UCO with methanol (molar ratio 6:1 methanol:FFA) using H₂SO₄ (1-2 wt%) as catalyst at 60°C for 1 hour. This converts FFAs to esters.
- Catalyst Check: If yield remains low, inspect catalyst activity. Sulfided metal catalysts can deactivate due to water and oxygenates. Consider catalyst regeneration (controlled oxidative treatment followed by re-sulfidation) or replacement.
- Process Parameter Optimization: Ensure hydroprocessing conditions are sufficient: typical ranges are 300-400°C and 50-150 bar H₂ pressure.
FAQ 2: How do I address catalyst coking and rapid deactivation in the catalytic pyrolysis step for Bio-Thermochemical Jet (BTJ) fuel production?
- Answer: Coke formation on zeolite catalysts (e.g., HZSM-5) is inherent due to the polyaromatic nature of pyrolysis vapors. Rapid deactivation indicates excessive residence time, high temperature, or unsuitable catalyst pore structure.
- Troubleshooting Protocol:
- In-Situ Regeneration: Integrate a fluidized-bed reactor with a continuous catalyst regenerator. Circulate coked catalyst to a regenerator unit operating at 550-700°C with air to burn off coke deposits.
- Parameter Adjustment: Reduce vapor residence time in the catalytic zone to <2 seconds. Modulate temperature to the lower end of the catalytic upgrading range (350-450°C).
- Catalyst Selection: Test hierarchical or mesoporous zeolites (e.g., mesoporous ZSM-5) to reduce diffusion limitations and coke formation. Compare deactivation rates against standard catalysts.
FAQ 3: What are the common contamination points in Alcohol-to-Jet (ATJ) fermentation that lead to poor isobutanol yield, and how can they be controlled?
- Answer: Contamination in ATJ's fermentation step (using engineered microbes like Clostridium or Saccharomyces) by bacteriophages or competing bacteria consumes sugar feedstock and produces inhibitors.
- Troubleshooting Protocol:
- Sterilization Validation: Verify autoclave performance (121°C, 15 psi, 20 minutes) for all media and feedstock. For continuous systems, implement sterile filtration (0.2 μm membrane) of feedstock streams.
- Process Monitoring: Use real-time PCR assays to detect specific microbial contaminants. A sudden pH drop or CO₂ evolution rate change can indicate infection.
- Antibiotic/Antimicrobial Protocol: In research-scale batches, incorporate approved antibiotics (e.g., virginiamycin for Gram-positive bacteria) at specified concentrations. Note: This may not be feasible at commercial scale.
- Culture Replenishment: Maintain a cryopreserved master cell bank and initiate new fermentations from this bank regularly to prevent genetic drift and contamination carryover.
FAQ 4: Why is the hydrogen consumption in my HEFA process significantly exceeding theoretical models?
- Answer: Excess H₂ consumption often points to side reactions: over-hydrogenation, methanation, or the hydrodeoxygenation (HDO) pathway dominating over decarboxylation/decarbonylation (DCO). DCO pathways consume less H₂.
- Troubleshooting Guide:
- Pathway Analysis: Analyze product slate via GC-MS. High proportions of n-alkanes (C15-C18) suggest HDO. High proportions of n-alkanes (C14-C17) suggest DCO is active.
- Catalyst Tuning: The choice of catalyst influences the pathway. Sulfided CoMo catalysts favor DCO, while NiMo favors HDO. Consider adjusting the catalyst formulation or metal loading.
- Pressure Optimization: Reduce H₂ partial pressure incrementally. While necessary for saturation, excessive pressure promotes over-hydrogenation and methanation (via CO/CO₂ hydrogenation), wasting H₂.
Table 1: Key Performance Indicators of Major Biomass SAF Pathways
| Pathway | Full Name | Typical Feedstocks | Technology Readiness Level (TRL) | Typical SAF Yield (wt%) | Key Challenge (for Infrastructure Adaptation) |
|---|---|---|---|---|---|
| HEFA | Hydroprocessed Esters and Fatty Acids | Oils (Soy, Canola, UCO, Tallow) | 8-9 (Commercial) | 60-75% | Feedstock consistency, H₂ supply, cold flow properties of final blend. |
| FT-SPK | Fischer-Tropsch Synthetic Paraffinic Kerosene | Lignocellulosic Biomass, MSW | 8 (First Commercial) | 25-50% | High capital cost, syngas purification, complex gasification infrastructure. |
| ATJ | Alcohol-to-Jet | Sugars, Starches, Lignocellulosic Sugars | 7-8 (Demo/Commercial) | 50-70% (from alcohol) | Alcohol purity, oligomerization catalyst lifetime, water removal. |
| CHJ | Catalytic Hydrothermolysis Jet | Fatty Acids, Oils | 6-7 (Demo) | 60-65% | High-pressure, high-temperature reactor design, hydrotreating severity. |
| FT-SPK/A | FT-SPK with Aromatics | Lignocellulosic Biomass, MSW | 5-6 (Pilot/Demo) | 20-45% | Selective aromatics synthesis to meet jet fuel spec D1655. |
Table 2: Experimental Results from HEFA Hydroprocessing of Model Compound (Methyl Oleate)
| Experiment # | Catalyst | Temperature (°C) | Pressure (bar H₂) | Liquid Hourly Space Velocity (h⁻¹) | Conversion (%) | Selectivity to C18 (n-Octadecane) (%) |
|---|---|---|---|---|---|---|
| 1 | Sulfided NiMo/γ-Al₂O₃ | 350 | 50 | 1.0 | 99.8 | 88 |
| 2 | Sulfided NiMo/γ-Al₂O₃ | 350 | 80 | 1.0 | ~100 | 92 |
| 3 | Sulfided CoMo/γ-Al₂O₃ | 350 | 50 | 1.0 | 99.5 | 76* |
| 4 | Sulfided NiMo/γ-Al₂O₃ | 300 | 50 | 1.0 | 85.2 | 95 |
*Higher proportion of C17 (heptadecane) from DCO pathway observed.
Experimental Protocols
Protocol 1: Bench-Scale HEFA Hydrotreating of Lipid Feedstocks Objective: To convert triglyceride feedstock into renewable paraffins suitable for SAF. Materials: Fixed-bed tubular reactor (SS316), HPLC pump, gas mass flow controllers, liquid product condenser, high-pressure separator. Procedure:
- Feedstock Prep: Pre-treat oil feedstock via filtration (1 µm) and drying (120°C under N₂).
- Catalyst Loading: Load 5-10 cc of sulfided NiMo/Al₂O₃ catalyst (60-80 mesh) into reactor. Dilute with inert SiC.
- Reactor Start-up: Pressurize system to 30 bar under N₂. Heat to 150°C, then switch N₂ to H₂. Set H₂ flow to desired space velocity (e.g., 1000 NL/L-feed).
- Reaction: Heat to target temperature (300-400°C) and pressure (50-150 bar). Introduce liquid feed via HPLC pump at desired LHSV (0.5-2 h⁻¹).
- Product Collection: Collect liquid effluent in a cooled high-pressure separator. Separate gas (analyze online via GC) and liquid.
- Analysis: Analyze liquid product via Simulated Distillation (ASTM D2887) and comprehensive GC-MS for hydrocarbon distribution.
Protocol 2: Catalytic Upgrading of Pyrolysis Vapors for BTJ (Micro-Reactor Setup) Objective: To deoxygenate biomass pyrolysis vapors over a zeolite catalyst to produce hydrocarbon intermediates. Materials: Micro-pyrolyzer (e.g., Pyroprobe), catalytic bed reactor attachment, quartz wool, GC-MS with FID. Procedure:
- Catalyst Prep: Load 2 mg of HZSM-5 catalyst (SiO₂/Al₂O₃ = 30) into a quartz reactor bed. Secure with quartz wool.
- Biomass Loading: Load 500 µg of ground pine biomass (100 mesh) into a separate pyroprobe cup.
- System Setup: Connect the catalytic bed directly to the pyroprobe interface. Set GC-MS inlet line to 300°C.
- Pyrolysis/Catalysis: Program pyroprobe to heat biomass at 1000°C/s to 500°C, hold for 20s. Simultaneously, heat catalytic bed to desired temperature (e.g., 550°C).
- Vapor Transfer: Pyrolysis vapors are swept by He carrier gas (1 mL/s) directly over the catalyst bed.
- Analysis: Upgraded vapors enter GC-MS for separation. Quantify major aromatic hydrocarbons (benzene, toluene, xylene, naphthalenes) via FID.
Visualizations
Title: HEFA-SAF Production Workflow
Title: ATJ-SAF Conversion Pathway Steps
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Biomass SAF Pathway Research
| Item | Function/Application | Example Product/Specification |
|---|---|---|
| Sulfided Hydrotreating Catalyst | Deoxygenation and hydrogenation of lipids in HEFA. | NiMo/γ-Al₂O₃ or CoMo/γ-Al₂O₃, presulfided, 1/16" extrudates. |
| HZSM-5 Zeolite Catalyst | Catalytic cracking and aromatization for pyrolysis vapor upgrading (BTJ). | Zeolite, SiO₂/Al₂O₃ ratio 23-80, 50-80 mesh powder. |
| Model Compound | Simplifying reaction studies for mechanism understanding. | Methyl oleate (for HEFA), Guaiacol (for pyrolysis upgrading). |
| High-Pressure Batch Reactor | Screening catalysts and conditions for hydroprocessing. | 100 mL Parr reactor, Hastelloy C-276, with gas injection stirrer. |
| Analytical Standard - Paraffins | Quantifying hydrocarbon yields in final SAF blendstock. | C8-C20 n-Alkane Standard Mix for GC calibration. |
| Solid Acid Catalyst | For alcohol dehydration step in ATJ pathway. | γ-Alumina, high surface area (>150 m²/g), acidic. |
| Gas Chromatography System | Detailed hydrocarbon analysis (SimDis, speciation). | GC with FID and capillary column (e.g., DB-1ms). |
| Fermentation Strain | Producing alcohols from sugars for ATJ research. | Engineered S. cerevisiae for isobutanol production. |
Technical Support Center: Troubleshooting & FAQs
Context: This support content is framed within the broader research thesis: "Infrastructure Adaptation for Biomass SAF Handling and Distribution: Mitigating risks posed by variable fuel chemistry." It addresses critical property-related challenges for researchers and development professionals.
Frequently Asked Questions (FAQs)
Q1: Our biomass-derived SAF sample showed unexpected viscosity increase and particulate formation after storage. What is the likely cause? A: This is commonly linked to thermal instability and auto-oxidation. The high oxygen content in many biomass-derived intermediates (e.g., carboxylic acids, alcohols) can lead to polymerization and condensation reactions over time, especially when trace metals are present. Ensure samples are stored under inert atmosphere (N2) at recommended temperatures (-20°C for long-term storage of reactive intermediates). Analyze oxygen content via ASTM D5622 or elemental analysis to gauge reactivity potential.
Q2: During filtration of a bio-oil fraction, the filter media rapidly clogged. The material also felt warm to the touch. What should we do? A: This indicates an exothermic reaction likely due to the hydroscopicity (moisture absorption) of the material. Bio-derived compounds with high oxygen functionality (e.g., sugars, levoglucosan) can rapidly absorb atmospheric moisture during handling, leading to swelling, hydrolysis, or heat release. Immediate Protocol: 1) Perform all handling in a controlled humidity environment (<20% RH). 2) Pre-dry filter media. 3) Use a chilled filtration apparatus. Consider characterizing hydroscopicity via dynamic vapor sorption (DVS) analysis.
Q3: Our analytical results for oxygen content vary significantly between duplicate samples. How can we improve reproducibility? A: Variability often stems from sample aging and exposure. High-oxygen compounds are frequently hygroscopic and reactive. Protocol for Representative Sampling: 1) Use airtight, sealed vials with PTFE septa. 2) Purge the vial headspace with argon before and after sampling. 3) Perform analysis immediately after extraction. If delay is unavoidable, store samples at -80°C with desiccant. The Karl Fischer titration method (ASTM E203) for water content should be run in parallel to correct for absorbed moisture.
Q4: We observed pressure buildup in sealed vials containing our SAF intermediate during a thermal stability test (80°C). What does this signify? A: Pressure buildup is a direct indicator of thermal decomposition, releasing low molecular weight gases (CO, CO2, CH4). This is a critical safety hazard for storage and transport infrastructure. Required Action: 1) Immediately depressurize in a fume hood. 2) Conduct a controlled Thermal Stability Test using a Closed Pressure Vessel Test (e.g., modified ASTM D7094). Monitor pressure over time at various temperatures to establish safe handling thresholds.
Table 1: Representative Property Ranges for Biomass-Derived Intermediates & SAF Blends
| Compound/Blend Type | Typical Oxygen Content (wt%) | Decomposition Onset Temp. (°C) | Moisture Uptake (DVS, % w/w @ 60% RH) | Recommended Max Storage Temp. (°C) |
|---|---|---|---|---|
| Fast Pyrolysis Bio-Oil | 35 - 45 | 80 - 90 | 15 - 25 | -25 |
| Hydroprocessed Esters and Fatty Acids (HEFA) | <1 | ~300 | <0.1 | 25 |
| Lignocellulosic Sugar Stream | ~50 | 110 - 130* | 20 - 30 | -80 |
| Alcohol-to-Jet (ATJ) Intermediate | 15 - 25 | 180 - 200 | 5 - 10 | 5 |
| Fully Upgraded Biomass SAF | <0.5 | >230 | <0.05 | Ambient |
*Decomposition refers to dehydration/oligomerization, not combustion.
Experimental Protocols
Protocol 1: Determining Effective Oxygen Content via Elemental Analysis & Subtraction Objective: Calculate the reactive oxygen content excluding bound water.
- Dry the sample over P2O5 in a desiccator for 72 hours.
- Perform Elemental Analysis (CHNS/O) using a combustion analyzer (e.g., PerkinElmer 2400). Record weight percentages of Carbon (C), Hydrogen (H), Nitrogen (N), and Sulfur (S).
- Perform Karl Fischer Titration (ASTM E203) on a separate aliquot to determine water content (H2O wt%).
- Calculate: Oxygen (wt%) = 100% - (C% + H% + N% + S% + Ash%). Reactive Oxygen ≈ Calculated O% - (H2O% * 0.89). Record both values.
Protocol 2: Accelerated Thermal Stability Assessment (Closed Ampoule Method) Objective: Visually assess compatibility and decomposition under accelerated aging.
- Load 5 mL of sample into a clean, dry glass ampoule (10 mL capacity).
- Freeze the sample in liquid nitrogen.
- Seal the ampoule under vacuum using an oxygen torch.
- Place sealed ampoules in pre-heated ovens at set temperatures (e.g., 40°C, 80°C, 120°C).
- Monitor: Remove ampoules at 1, 3, 7, 14-day intervals. Visually inspect for color change, phase separation, and gas formation (bubbles). Filter contents through a pre-weighed 0.45 µm filter to measure insoluble gum formation.
Protocol 3: Dynamic Vapor Sorption (DVS) for Hydroscopicity Profile Objective: Quantify moisture uptake as a function of relative humidity (RH).
- Pre-dry sample in the DVS instrument at 0% RH and 25°C until equilibrium (dm/dt < 0.002%/min).
- Program a sorption isotherm: Step RH from 0% to 95% in 10% increments, holding at each step until equilibrium (typical dm/dt threshold: 0.002%/min).
- Program a desorption isotherm: Step RH back down from 95% to 0%.
- Analyze: Plot mass change (%) vs. RH. Report equilibrium moisture uptake at 60% RH and any hysteresis between sorption/desorption curves.
Visualizations
Diagram Title: Thermal Stability Test Workflow
Diagram Title: Interplay of Critical Biomass SAF Properties
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Biomass SAF Property Analysis
| Item | Function & Rationale |
|---|---|
| Anhydrous Sodium Sulfate (Na2SO4) | Common drying agent for organic samples; removes trace water before analysis to prevent skewed oxygen/hydroscopicity results. |
| Inert Atmosphere Glove Box (N2 or Ar) | Provides O2- and H2O-free environment for sampling, weighing, and preparing reactive, oxygen-sensitive, or hygroscopic intermediates. |
| Sealed Pressure Vessels (e.g., Swagelok ampoules) | Critical for safe thermal stability testing; allows visual monitoring of gas formation and pressure buildup from decomposition. |
| Karl Fischer Titrator with Oven Module | Precisely measures total water content (free and bound) via coulometric or volumetric titration; essential for hydroscopicity assessment. |
| PTFE-Lined Septa & Vials | Provides an inert, non-reactive seal for sample storage; prevents leaching and interaction that could catalyze decomposition. |
| Dynamic Vapor Sorption (DVS) Instrument | Quantifies moisture uptake/loss isotherms; key for modeling hygroscopic behavior and its impact on fuel handling systems. |
| Differential Scanning Calorimeter (DSC) | Measures heat flow to determine decomposition onset temperature and oxidative stability (e.g., via ASTM E2009). |
Technical Support Center: Biomass SAF Handling & Distribution Research
Welcome, Researchers. This center addresses common experimental challenges when adapting existing Jet-A1 infrastructure for biomass-derived Sustainable Aviation Fuel (SAF). Our troubleshooting guides and FAQs are framed within the thesis that legacy systems require significant, non-trivial adaptation for novel fuel chemistries.
Frequently Asked Questions (FAQs)
Q1: During cold soak filtration tests, we observe rapid filter plugging with our Hydroprocessed Esters and Fatty Acids (HEFA) sample, unlike with conventional Jet-A1. What is the likely cause? A: This is a common incompatibility with legacy cold-weather handling infrastructure. Biomass-derived SAF, particularly HEFA and Alcohol-to-Jet (ATJ) fuels, can contain trace oxygenates and different hydrocarbon profiles (higher paraffinic content) that crystallize at different temperatures and morphologies than conventional fuel. This leads to faster filter blocking. Protocol: Replicate ASTM D5972/IP 435 for "Fuel System Icing Inhibitor and Filterability," but extend the test to include a slow cooling cycle (0.5°C/min) from 20°C to -40°C while monitoring pressure differential. Compare crystallization curves.
Q2: Why does our experimental blend (50% SAJF-SPK) cause swelling and reduced seal integrity in our laboratory-scale peristaltic pumps after 72 hours? A: Current Jet-A1 infrastructure uses elastomers (e.g., nitrile rubber) compatible with aromatic hydrocarbons. Many SAF pathways produce near-zero aromatic, fully saturated fuels. This alters the swelling balance. The lack of aromatic compounds leads to shrinkage and hardening of some elastomers, causing leaks. Protocol: Conduct a static immersion test per ASTM D471. Test O-rings of Viton, nitrile rubber, and fluorosilicone in 100% SAF and 50/50 blends vs. Jet-A1. Measure mass change, volume change, and durometer hardness at 24h intervals.
Q3: Our analysis shows unexpected trace metal contamination (Ca, K) in the fuel after storage in a repurposed Jet-A1 tank. Is this from the fuel or the system? A: Likely system leaching or biofilm interaction. Biomass SAF may have different solvent properties and can contain trace oxygenated species that interact with tank sediments or microbial communities (fungi, bacteria) that were dormant on Jet-A1. These microbes can mobilize metals. Protocol: Implement a tank simulation test. Add 1L of test fuel to clean glass containers with coupons of typical tank materials (steel, aluminum with typical coatings). Age at 30°C for 4 weeks. Analyze fuel weekly via ICP-MS for metals and perform microbial counts using culture media for Hormoconis resinae and Pseudomonas aeruginosa.
Q4: When testing fuel thermal stability (ASTM D3241 "JFTOT"), our co-processed SAF blend shows acceptable pressure drop but increased tube deposit colors. What does this indicate? A: This indicates different deposit chemistry. Conventional Jet-A1 deposits are often carbonaceous. SAF deposits may involve polymerization of trace olefins or oxygenates under thermal stress, forming varnish-like deposits. The color difference (brown vs. black) is key. Protocol: Perform modified JFTOT per ASTM D3241. After the test, use solvent washing (tetrahydrofuran) to collect deposits from the test tube. Analyze via FTIR and SEM-EDS to compare elemental (C, O, N) composition of deposits from SAF vs. conventional fuel.
Table 1: Key Property Contrasts Driving Infrastructure Adaptation
| Property | Conventional Jet-A1 (Typical) | HEFA-SPK (Typical) | Infrastructure Challenge |
|---|---|---|---|
| Aromatic Content | 8-25% (vol) | <0.5% (vol) | Elastomer shrinkage, seal failure |
| Sulfur Content | <1000 ppm | <1 ppm | Reduced lubricity, potential corrosion |
| Energy Density (MJ/L) | ~35.5 | ~34.2 (~3.7% lower) | Range/payload calculations, fuel heating may be needed |
| Distillation Curve (T50) | ~205°C | ~185°C (lighter) | Vapor lock risk, different pump design needs |
| Thermal Stability Deposit | Carbonaceous | Varnish/Oxidative | Different fouling mechanisms in heat exchangers |
Table 2: Common Material Compatibility Test Results
| Material | Exposure (100% HEFA, 30°C, 4 weeks) | Result (Change vs. Jet-A1 Baseline) |
|---|---|---|
| Nitrile Rubber (NBR) | Volume Change | -5% to -8% (Shrinkage) |
| Viton (FKM) | Volume Change | +1% to +2% (Stable) |
| Carbon Steel (Coated) | Corrosion Rate | No significant change |
| Copper Alloy | Corrosion Rate | Slight increase (0.002 mm/yr) |
| Polypropylene | Tensile Strength Loss | <2% (Acceptable) |
Experimental Protocols
Protocol 1: Modified Cold Soak Filtration Test for SAF Crystallization Behavior
- Objective: Determine the low-temperature filterability of SAF blends.
- Materials: 500 mL fuel sample, automated cold soak bath, 1.6 µm mesh filter, pressure transducer, data logger.
- Procedure: a. Pre-cool fuel to 20°C. b. Install filter assembly and prime with fuel. c. Place entire assembly into bath programmed to cool to -40°C at 0.5°C/min. d. Initiate flow at 20 mL/min once bath reaches -25°C. e. Continuously record pressure differential across the filter until a 105 kPa limit is reached or temperature stabilizes. f. Plot ΔP vs. Temperature and Time. Compare inflection points with Jet-A1 control.
Protocol 2: Static Immersion for Elastomer Compatibility
- Objective: Quantify volume change and hardness of sealing materials in SAF.
- Materials: O-rings (NBR, FKM, EPDM), analytical balance, volume displacement apparatus (e.g., via water), durometer.
- Procedure: a. Weigh and measure initial volume of O-rings (using buoyancy method). b. Record initial durometer reading. c. Immerse O-rings in sealed jars containing test fuel and control (Jet-A1). Store at 40°C to accelerate aging. d. At intervals (24h, 72h, 168h), remove O-rings, blot dry, and repeat measurements. e. Calculate % change in mass and volume. Note hardness change.
Mandatory Visualization
Title: SAF & Legacy Infrastructure Interaction Pathway
Title: Material Compatibility Test Workflow
The Scientist's Toolkit: Research Reagent Solutions
| Item / Reagent | Function in SAF Infrastructure Research |
|---|---|
| Certified Reference Fuels | Jet-A1 (ASTM D1655) & SAFs (e.g., HEFA-SPK, FT-SPK). Provide baseline for all comparative testing. |
| Material Coupon Kits | Pre-cut discs/rods of carbon steel, aluminum, elastomers (NBR, FKM), and composites. For standardized immersion tests. |
| Cold Soak Filtration Apparatus | Automated system per ASTM D5972/IP 435. Essential for low-temperature performance validation. |
| JFTOT (Jet Fuel Thermal Oxidation Tester) | Standard apparatus (ASTM D3241) for assessing thermal stability and deposit formation. |
| Microbial Culture Media | Specific for hydrocarbon-utilizing fungi (Hormoconis resinae) and bacteria. Monitors biocontamination in storage simulations. |
| ICP-MS Calibration Standards | For trace metal analysis (Ca, K, Na, Mg, Fe) to detect corrosion products or system leaching. |
| FTIR with ATR Attachment | For chemical analysis of deposits, identifying oxygenated species or polymers formed during stress tests. |
| Digital Durometer | Measures Shore A hardness of elastomers before/after fuel exposure, quantifying material degradation. |
Technical Support Center
Troubleshooting Guide: Common Material Failure Scenarios
Scenario 1: Elastomer Seal Swelling and Leakage in SAF Blend Service
- Observed Issue: O-ring or gasket exhibits significant volumetric swelling (>25%), loss of durometer hardness, and subsequent leak in a valve or flange connection handling hydroprocessed esters and fatty acids (HEFA)-SAF blended with conventional jet fuel.
- Root Cause: High aromatic or ketone content in certain SAF blending components, or trace oxygenates from processing, are incompatible with nitrile (NBR) or ethylene-propylene diene monomer (EPDM) elastomers.
- Immediate Action:
- Isolate and depressurize the system.
- Replace failed seal with a fluorocarbon (FKM/Viton) or perfluoroelastomer (FFKM) grade specifically pre-tested for the exact SAF blend.
- Flush the local line segment to remove degraded polymer residues.
- Preventive Protocol: Implement the Static Immersion & Mechanical Property Test (detailed below) for all elastomers prior to system commissioning.
Scenario 2: Adhesive/Sealant Degradation at Biomass Feedstock Interface
- Observed Issue: Polysulfide or silicone-based sealants in sampling ports or sight glasses become soft, discolored, or show adhesive failure when exposed to biomass pyrolysis vapors or liquid intermediates.
- Root Cause: Acidic compounds (e.g., organic acids, phenolics) or trace sulfur species in pyrolysis bio-oil catalyze sealant depolymerization.
- Immediate Action:
- Safely collect and contain any leaked material.
- Mechanically remove degraded sealant. Surface cleaning with isopropanol may be required.
- Re-seal using a high-performance, chemically resistant sealant like a two-part epoxy or fluorosilicone, ensuring proper surface preparation.
- Preventive Protocol: Specify sealants based on Chemical Compatibility Matrices for the specific process stream (see Data Tables).
Scenario 3: Corrosion of Legacy Carbon Steel in Wet SAF/Blend Environments
- Observed Issue: Pitting or uniform corrosion on internal surfaces of legacy carbon steel transfer lines or tanks, particularly at vapor/liquid interfaces, when handling SAF blends with higher water solubility.
- Root Cause: Residual oxygen and organic acids in the fuel blend, combined with entrained water from new feedstock pathways, create a corrosive microenvironment.
- Immediate Action:
- Inspect via non-destructive testing (ultrasonic thickness testing).
- For operational continuity, consider applying a compatible internal lining or corrosion inhibitor certified for aviation fuel.
- Plan for replacement with 300-series stainless steel (e.g., 304/316L) or coated aluminum.
- Preventive Protocol: Implement rigorous Water Separation Index Plus (WSI+) monitoring and install sacrificial anodes or cathodic protection in tanks.
Frequently Asked Questions (FAQs)
Q1: Our lab-scale reactor uses Viton O-rings. Are these safe for all potential biomass-derived intermediates? A1: Not universally. While fluoroclastomers (FKM) like Viton offer broad chemical resistance, certain emerging biomass intermediates like methyl esters (FAME) or high-concentration organic acids can cause excessive swelling or chemical attack on standard FKM grades. You must specify Low-Temperature FKM (LT-FKM) or ETP (Extended Temperature Performance) grades and validate them via immersion testing against your specific process stream.
Q2: We are retrofitting an old pilot plant originally for petroleum. What is the highest priority material compatibility check for handling 100% HEFA-SAF? A2: The highest priority is a comprehensive audit of all elastomeric components (seals, hoses, gaskets) and non-metallic coatings/lining. Legacy systems commonly use nitrile or neoprene, which have high failure risk. Metals like carbon steel may be acceptable if the fuel meets stringent acidity and water content specs, but elastomers will likely require systematic replacement before introduction of SAF.
Q3: Is there a standardized test to quickly screen sealant compatibility with new SAF formulations? A3: Yes, the modified ASTM D471 test is the benchmark. Immerse standardized coupons of the sealant material in the fuel blend at elevated temperature (e.g., 60°C) for a defined period (e.g., 28 days). Measure changes in volume, mass, hardness, and tensile strength. Key acceptance thresholds are detailed in the Experimental Protocol section.
Q4: Can we use copper or brass fittings in SAF distribution systems? A4: It is strongly discouraged. Copper and its alloys are known catalysts for oxidation and degradation of hydrocarbon fuels, potentially leading to gum formation and filter plugging. This is exacerbated in biofuels which may contain polar compounds. Specify stainless steel or aluminum for all wetted parts.
Table 1: Elastomer Volume Swell (%) in 50/50 Blend with Conventional Jet A-1 (28-Day Immersion at 60°C)
| Elastomer Type | HEFA-SAF | FT-SPFK-SAF | AtJ-SAF (Alcohol-to-Jet) |
|---|---|---|---|
| Nitrile (NBR) - 70 durometer | +32% | +18% | +45% (Failed) |
| Hydrogenated Nitrile (HNBR) | +12% | +8% | +28% |
| Ethylene Propylene (EPDM) | +8% | +5% | +65% (Failed) |
| Fluorocarbon (FKM) - Standard | +5% | +2% | +4% |
| Perfluoroelastomer (FFKM) | <+1% | <+1% | <+1% |
Table 2: Corrosion Rate of Legacy Metals in Wet SAF Environment (mpy*)
| Metal Alloy | Deionized Water Saturated | 100 ppm Acetic Acid Added | 30 ppm Water + 5 ppm O₂ |
|---|---|---|---|
| Carbon Steel (A106) | 2.1 | 15.7 | 8.3 |
| 304 Stainless Steel | <0.1 | 0.5 | <0.1 |
| 316 Stainless Steel | <0.1 | <0.1 | <0.1 |
| Aluminum 6061 | 0.3 | 12.4 | 1.2 |
| Copper C110 | 0.2 | 9.8 | 1.5 |
*mpy = mils (0.001 inch) per year
Experimental Protocols
Protocol 1: Static Immersion Test for Elastomer & Sealant Compatibility (Modified ASTM D471/D7216)
- Objective: Quantify the physical and mechanical degradation of non-metallic materials upon exposure to SAF blends.
- Materials: Test fuel blend, elastomer/sealant coupons (e.g., 50mm x 25mm x 2mm), sealed glass jars, temperature-controlled oven, analytical balance, durometer, calipers.
- Methodology:
- Prepare and weigh/measure three control coupons.
- Immerse three test coupons in fuel within sealed jars, ensuring no air headspace.
- Place jars in oven at 60°C ± 2°C for 28 days.
- Remove coupons, gently blot surface liquid, and immediately weigh and measure dimensions.
- Measure durometer hardness after 30-minute cooling period.
- Calculate percent change in volume, mass, and hardness relative to controls.
- Acceptance Criteria: For static seals, volume swell >25% or hardness change >15 points typically indicates incompatibility. Tensile strength loss should be <30%.
Protocol 2: Dynamic Seal Test for Rotary or Reciprocating Motion
- Objective: Evaluate seal performance under simulated service conditions (friction, temperature cycling).
- Materials: Test fixture (e.g., rotating shaft seal housing), SAF blend, thermocouples, torque sensor, leak detection system.
- Methodology:
- Install test seal in fixture. Fill reservoir with SAF blend.
- Cycle temperature between 20°C and 80°C over 8-hour periods.
- Operate shaft at typical service speed (e.g., 100-500 rpm).
- Monitor torque (indicative of friction/stiction) and check for leakage hourly.
- Run test for 100-500 hours, then inspect seal for wear, cracking, or permanent set.
Diagrams
Title: Material Failure Troubleshooting Workflow
Title: Fuel Components & Material Degradation Pathways
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Compatibility Testing
| Item | Function/Description | Critical Consideration for SAF Research |
|---|---|---|
| Fluorocarbon (FKM) O-Ring Kit | Assortment of seals in various grades (e.g., standard, LT, ETP). Used for rapid replacement and testing in fluidic systems. | Verify polymer grade (e.g., GLT, GFLT) for low-temperature and ester resistance. |
| Perfluoroelastomer (FFKM) Coupons | Gold-standard elastomer for immersion testing. Serves as a control or benchmark for aggressive chemical environments. | Extremely high cost limits use to critical static seals only. |
| 316L Stainless Steel Tubing & Fittings | Inert wetted surfaces for constructing sample loops, transfer lines, and reactor sections. | Preferred over 304 for chloride and acid resistance in biomass-derived streams. |
| Water Separation Index Plus (WSI+) Test Kit | Quantifies the ability of fuel to release entrained water. Critical for corrosion prediction. | SAF blends can show different surfactant behavior than conventional fuel, making WSI+ essential. |
| Microbalance (±0.01 mg) | Precisely measures mass change of metal coupons during corrosion tests or polymer samples during immersion. | Requires controlled environment (humidity, temperature) for accurate long-term testing. |
| Portable Durometer (Shore A Scale) | Measures hardness of elastomeric materials before and after exposure to assess degradation. | Must use same measurement point on coupon pre- and post-test. Follow ASTM D2240. |
| Anaerobic Chamber Glove Box | Creates oxygen-free environment for preparing and sealing test samples to isolate the effect of fuel alone, excluding oxidation. | Critical for studying the intrinsic compatibility of anaerobic processing intermediates. |
| Inductively Coupled Plasma (ICP) Optical Emission Spectrometer | Analyzes metal ion content in fuel after exposure to metals, quantifying trace leaching and corrosion. | Detects ppm-level leaching of catalyst metals (e.g., Cu) that can fuel instability. |
Technical Support Center: Troubleshooting & FAQs for SAF Research
FAQs
Q1: During the analysis of a synthetic paraffinic kerosene (SPK) blend, our results show a deviation from the specified properties in ASTM D7566 Annex A5 (for Hydroprocessed Esters and Fatty Acids - HEFA). What are the most common root causes? A1: Common root causes are:
- Feedstock Contamination: Trace elements (e.g., Na, K, Ca) from biomass feedstocks can poison hydroprocessing catalysts, leading to off-spec products. Check feedstock purity via ICP-MS.
- Incomplete Deoxygenation: Insufficient hydroprocessing severity can leave oxygenates, affecting thermal stability (D3241) and lowering the Net Heat of Combustion (D4809). Verify via FT-IR for C=O stretches.
- Isomerization Control: Improper branching control affects freeze point (D5972, D7153) and viscosity (D445). Analyze using GCxGC for detailed hydrocarbon speciation.
Q2: When blending SAF with conventional Jet A-1 to meet D7566 requirements, we encounter phase separation or haze. How do we troubleshoot this? A2: This indicates a compatibility failure per ASTM D7566, Section 6. Haze often results from:
- High Melting Point Components: Saturated linear paraffins from HEFA can precipitate. Measure the freezing point of the neat SPK (D5972) before blending.
- Trace Polar Compounds: Residual alcohols, acids, or mono-glycerides. Conduct a detailed hydrocarbons analysis (DHA) per D2425.
- Water Contamination: Use Karl Fischer titration (D6304) on both blend components. Ensure storage tanks and transfer lines are dry and dedicated.
Q3: Our corrosiveness testing (D130) on a novel catalytic hydrothermolysis (CH) SAF shows elevated copper strip scores. What does this imply for infrastructure? A3: Elevated scores suggest the presence of corrosive sulfur or acids. This has direct infrastructure implications:
- Immediate Risk: Corrosion of copper alloys in fuel system components (e.g., heat exchangers, seals).
- Long-term Impact: Potential for particulate generation, filter plugging, and increased wear. You must investigate the production process:
- Check for incomplete sulfur removal from catalyst or feedstock.
- Analyze for organic acids via acid number testing (D3242).
Q4: How do the aromatic content requirements in D7566 (now supplied by the synthetic aromatic hydrocarbons - SAH) impact material compatibility in existing distribution systems? A4: The mandated switch from natural aromatics to synthesized ones (e.g., alkylbenzenes) affects elastomer swell. Existing O-rings and gaskets (e.g., nitrile, neoprene) may shrink or harden, causing leaks. The troubleshooting protocol is:
- Perform swell tests (D471) on all elastomers in contact with the SAH/SPK blend.
- Systematically inspect and potentially replace seals with fluoroelastomers (e.g., Viton) that are compatible with low-aromatic fuels.
Experimental Protocol: Assessing SAF Blend Compatibility with Infrastructure Elastomers (Per ASTM D471 & D7566)
Objective: To evaluate the volumetric swell and hardness change of common fuel system elastomers when exposed to a D7566-qualified SAF blend versus conventional Jet A-1.
Materials:
- Test Fuels: Neat SPK (HEFA), Conventional Jet A-1 (reference), D7566 Blend (Max 50% SPK).
- Elastomer Coupons: Nitrile rubber (NBR), Fluoroelastomer (FKM), Epichlorohydrin (ECO) – cut to ASTM D471 specifications.
- Equipment: Analytical balance (±0.1 mg), Hardness durometer (Type A), Temperature-controlled immersion bath, Micrometer.
Methodology:
- Baseline Measurement: Weigh (W1), measure volume (V1) via fluid displacement, and record hardness (H1) for each coupon.
- Immersion: Immerse coupons in sealed vessels containing each test fuel. Condition at 40°C ± 2°C for 168 hours (1 week).
- Post-Test Measurement: Remove coupon, blot dry gently. Within 30 seconds, weigh (W2) and measure hardness (H2). After 30 minutes cooling, measure volume (V2).
- Calculation:
- Volume Change (%) = [(V2 - V1) / V1] * 100
- Hardness Change (points) = H2 - H1
- Mass Change (%) = [(W2 - W1) / W1] * 100
- Analysis: Compare results against OEM specifications (typically max +25% to -5% volume change, max ±10 points hardness change).
Signaling Pathway for SAF Specification Development and Infrastructure Impact
SAF Property to Infrastructure Impact Logic
Research Reagent Solutions & Essential Materials Toolkit
| Item/Category | Function/Application in SAF Research | Example Product/Specification |
|---|---|---|
| Certified Reference Materials | Calibrating instruments for D7566 test methods (e.g., DHA, freeze point). | NIST SRM 2770 (Jet A-1), Paraffin/Isoparaffin/Aromatic mix for GC. |
| Hydroprocessing Catalyst | For HEFA pathway lab-scale studies; deoxygenates and isomerizes lipids. | Sulfided NiMo/Al₂O₃ or Pt/SAPO-11 catalysts. |
| Specialty Elastomer Coupons | Material compatibility testing per D471 for seals and hoses. | NBR, FKM, ECO sheets, cut to D471 dimensions. |
| Solid Phase Extraction (SPE) Cartridges | Clean-up of SAF samples for trace contaminant analysis (metals, acids). | Silica, Aminopropyl, or C18 cartridges. |
| Calorimeter Standards | Validating Net Heat of Combustion (D4809) measurements. | Benzoic acid combustion calorimetry standard. |
| Water Standard for KF | Precise calibration for water content analysis (D6304). | Hydranal-Water Standard 1.00 mg/mL. |
| Copper Strips | Assessing corrosivity per D130. | ASTM D130 Polished Copper Strips. |
Data Summary: Key ASTM D7566 Property Limits and Infrastructure Concerns
| ASTM Property | Test Method | Typical Limit | Direct Infrastructure Implication |
|---|---|---|---|
| Aromatic Content | D6379 / D1319 | 8.0 - 25.0 vol% (by SAH) | Elastomer swell control; seal compatibility. |
| Distillation (T50-T10) | D2887 / D7344 | Report | Volatility for pump operation & cold start. |
| Fatty Acid Methyl Esters (FAME) | D7806 | Max 5 mg/kg (ppm) | Material degradation, filter blockage. |
| Thermal Stability (JFTOT) | D3241 | Max 25 mm Hg @ 260°C or 325°C | Deposit formation in heat exchangers & injectors. |
| Electrical Conductivity | D2624 | Min 50 pS/m (with static dissipator) | Static accumulation risk in pipelines & tankers. |
| Metals (Na+K+Ca) | ICP-MS (D8111) | Max 1.0 mg/kg total | Turbine corrosion and deposits. |
| Freeze Point | D5972 / D7153 | Max -40°C / -47°C | Flow assurance in cold climates & high altitude. |
Building the Supply Chain: Methodologies for Storage, Handling, and Distribution
Technical Support Center
Troubleshooting Guide
Q1: Our biomass blend, stored in a pilot-scale tank, is showing a rapid decrease in pH and increased viscosity. What is the likely cause and immediate action?
A: This is a classic indicator of microbial fermentation, likely from acid-producing bacteria or fungi. Immediate action:
- Stop the process and isolate the tank.
- Sample for analysis: Perform immediate microbial plating (see Protocol 1) and measure water content.
- Implement a short-term biocide treatment following manufacturer and safety guidelines for your specific biomass.
- Review tank conditions: Check and document temperature, aeration, and bottom-drain water accumulation.
Q2: We observe visible water layer separation at the bottom of our storage tank. How do we safely remove it without disturbing the biomass blend?
A: Water accumulation creates a microbial breeding ground. For safe removal:
- Use a water-finding paste on a dip stick to confirm the water layer depth.
- Utilize a fixed, bottom-mounted water draw-off leg with a sight glass. Open the valve slowly to drain only the aqueous phase.
- If no draw-off leg is installed, a floating suction pump can be carefully positioned at the oil-water interface. Never drain from the very bottom without continuous monitoring, as this can agitate the entire tank.
Q3: What inert gas blanketing is most effective for biomass blends, and how do we monitor oxygen ingress?
A: Nitrogen (N₂) is the standard for inert blanketing. Key steps:
- Maintain a positive pressure of 0.5-1.5 psi in the tank headspace.
- Use a pressure-vacuum vent valve (conserving vent) to maintain this blanket.
- Monitor oxygen ingress with a headspace oxygen analyzer (e.g., zirconia or laser-based). Target <0.5% O₂.
- Cost-effective alternative: For some blends, boiler-scrubbed CO₂ can be used if it does not affect feedstock chemistry.
Frequently Asked Questions (FAQs)
Q4: What are the critical parameters to log for preventative maintenance of a biomass storage tank?
A: Log the following parameters daily or per batch:
| Parameter | Target Range | Measurement Tool | Risk if Out of Range |
|---|---|---|---|
| Blend Temperature | < 20°C (or as blend-specific) | PT100 Thermowell | ↑ Microbial growth, ↑ Degradation |
| Headspace O₂ | < 0.5% | In-line Oxygen Analyzer | ↑ Oxidative & Microbial spoilage |
| Bottom Water Level | 0 cm (Trace only) | Water Finding Paste / Dip | Biofilm formation, Corrosion |
| pH | Blend-specific baseline | pH Probe / Sample Test | Early indicator of fermentation |
| Viscosity | Blend-specific baseline | In-line Viscometer | Indicator of polymerization or growth |
Q5: Which biocides are compatible with biomass intended for Sustainable Aviation Fuel (SAF) conversion pathways (e.g., HEFA, ATJ)?
A: Biocide selection must not poison downstream catalysts (e.g., hydrotreating). Consult your catalyst supplier. Commonly considered options:
| Biocide Type | Example | Key Consideration for SAF Pathways |
|---|---|---|
| Isothiazolinones | CMIT/MIT | Effective broad-spectrum; must ensure complete degradation pre-processing. |
| Oxidizing | Peracetic Acid | Breaks down to harmless by-products; can be corrosive to tank. |
| Quaternary Ammonium Compounds | Didecyl dimethyl ammonium chloride | May leave residues; extensive hydrotreating may be required. |
| Physical | UV Sterilization | No chemical residue; effective for clear, low-solids transfer lines. |
Q6: Can you recommend a protocol for sampling a tank to map microbial contamination gradients?
A: See Protocol 1 below.
Q7: How does tank material (e.g., stainless steel vs. coated carbon steel) impact microbial adhesion and biofilm formation?
A: Surface roughness is key. Electropolished 316L stainless steel provides the lowest roughness (< 0.8 µm) for easiest cleaning. Coated carbon steel (epoxy, phenolic) can be effective but is prone to pinhole defects and damage, creating niches for biofilm. Critical factor: The tank seam welding quality must be smooth and continuous.
Experimental Protocols
Protocol 1: Tank Sampling for Microbial Contamination Gradient Analysis
Objective: To systematically sample a storage tank to identify zones of high microbial load and water accumulation.
Materials:
- Sterile sample thieves (surface, mid-depth, bottom-drain)
- Sterile sample bottles (with neutralizing buffer if biocide is present)
- Water-finding paste
- Dip tape or calibrated dip stick
- GPS (Good Sampling Practice) labels
Methodology:
- Safety: Follow confined space and chemical exposure protocols.
- Zonal Sampling: Draw samples from three distinct zones:
- Surface/Biofilm: Use a sterile surface plate or thief from the top access port.
- Bulk Matrix: Use a zone sampler from the middle layer.
- Bottom/Sediment: Draw from the bottom drain valve before any water draw-off. If no drain, use a weighted sterile bottle opener.
- Water Layer: After taking the bottom product sample, use water-finding paste on a clean dip stick to measure water layer thickness. Draw a separate sample of this water layer for microbial and ionic analysis.
- Analysis: Process samples within 30 minutes for:
- Total Aerobic Microbial Count (TAMC) on TSA agar, incubated at 30°C for 3-5 days.
- Anaerobic & Sulfate-Reducing Bacteria (SRB) using specific vials or agar.
- pH and water content of each sample.
- Mapping: Log data against sampling coordinates (height from tank bottom) to create a contamination profile.
Protocol 2: Evaluating Biocide Efficacy in a Simulated Biomass Blend
Objective: To test the minimum inhibitory concentration (MIC) of a biocide against a microbial consortium isolated from a contaminated tank.
Materials:
- Isolated microbial consortium from Protocol 1.
- Sterile biomass blend (simulated or uncontaminated).
- Candidate biocide stock solution.
- 96-well microtiter plates.
- Spectrophotometer (OD600).
Methodology:
- Inoculum Prep: Suspend microbial consortium in sterile saline to 0.5 McFarland standard (~1.5 x 10^8 CFU/mL). Dilute 1:100 into sterile biomass blend.
- Biocide Dilution: Create a 2X serial dilution of the biocide in sterile blend across a 96-well plate.
- Inoculation: Add an equal volume of inoculated blend to each well, creating a 1X biocide concentration range. Include growth control (no biocide) and sterility control (no inoculum).
- Incubation & Reading: Seal plate and incubate at tank-relevant temperature (e.g., 25°C). Measure OD600 daily for 7 days.
- Analysis: The MIC is the lowest concentration that prevents a significant increase in OD600 compared to the sterility control. Confirm by plating from clear wells onto agar.
Visualizations
Title: Microbial Degradation Cycle in Biomass Storage Tanks
Title: Integrated Mitigation Strategy Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
| Item | Function & Relevance to Biomass Storage Research |
|---|---|
| Headspace Oxygen Analyzer (Zirconia type) | Continuous, real-time monitoring of O₂ levels in tank headspace to verify inert blanket efficacy. |
| Water-Finding Paste | Applied to a dip stick; changes color upon contact with water to accurately measure water layer bottom. |
| Dip Tape with Thermowell | For manual measurement of product level and temperature gradients within the tank. |
| Total Organic Carbon (TOC) Analyzer | Measures dissolved carbon in water draw-off samples, indicating microbial by-product leaching. |
| ATP Bioluminescence Assay Kit | Provides rapid (minutes) relative measure of viable microbial load on swab samples from tank walls. |
| Sterile, Sealed Sample Thief | Allows for aseptic sampling of solids and liquids from specific depths of a tank for microbial analysis. |
| Corrosion Coupons (Various alloys) | Small metal plates inserted into the tank headspace and liquid to monitor corrosion rates over time. |
| In-line Viscometer (Vibrating fork or inline capillary) | Provides continuous viscosity data as an early indicator of microbial spoilage or polymerization. |
Technical Support Center: Troubleshooting Guides and FAQs
FAQ 1: Dedicated Line Contamination
- Q: We observed unexpected lipid profiles in our hydroprocessed esters and fatty acids (HEFA) sample after using a dedicated line that previously carried petroleum-based distillate. What could be the cause?
- A: Residual adsorption/desorption is the likely culprit. Even dedicated lines require specific purging protocols when switching feedstocks with differing polarities. A "dedicated" line for biomass SAF must be cleared with a minimum of 5 line volumes of a compatible intermediate solvent (e.g., renewable diesel) before introducing a new biomass-derived batch. Failure to do so can lead to trace contamination altering final product specifications.
FAQ 2: Batch Sequencing Failure
- Q: Our simulated batch sequencing for Fischer-Tropsch wax and alcohol-to-jet (ATJ) intermediates resulted in off-spec blending at the interface. How can we improve demarcation?
- A: This indicates inadequate spacer volume or inappropriate spacer fluid properties. The spacer (e.g., a nitrogen bubble or a neutral, miscible fluid) must be sized based on pipeline diameter, length, and flow turbulence. Implement the following protocol:
Experimental Protocol: Optimal Spacer Volume Determination
- Objective: Determine the minimum spacer volume (
V_s) to prevent commingling between two successive batches (Batch A and B) in a horizontal pipeline. - Materials: Two non-reactive, miscible fluids with contrasting dyes (A=Blue, B=Clear), calibrated pump, transparent pipeline test section (Length L=10m, Diameter D=0.1m), high-resolution camera.
- Method:
a. Fill the line with Fluid A.
b. Inject a candidate spacer volume (
V_s_test) of an inert, low-density fluid (e.g., purified water if immiscible with test fluids). c. Initiate flow of Fluid B at a controlled rate (Q). d. Record the displacement along the pipeline at which the first visible trace of Fluid B appears in samples taken at the outlet. e. Calculate the theoretical dispersion length (L_d) using the empirical formula:L_d = 11.5 * D * (Re)^-0.2, whereReis the Reynolds number. f. The adequate spacer volume isV_s = π * (D/2)^2 * L_d. Iterate until experimental results confirm a sharp interface (<2% mixing by volume).
FAQ 3: Commingling Protocol Authorization
- Q: Our commingling protocol for blending bio-derived synthetic paraffinic kerosene (SPK) with conventional Jet A-1 was rejected by the logistics operator. What key data was missing?
- A: Logistics operators require validated compatibility and quality data. Your protocol must include a certified table from a recent (<6 months) simulated pipeline conditioning study, proving the mixture meets all ASTM D7566 Annexes. The most common omission is failure to demonstrate thermal oxidative stability (ASTM D3241) after simulated pipeline transit, which includes exposure to trace metals from line walls.
Data Presentation: Simulated Pipeline Conditioning Results for SPK/Jet A-1 Commingling
Table 1: Key Property Comparison Before and After Simulated Pipeline Transit (50/50 Blend)
| Property (Test Method) | Pre-Transit Specification | Post-Transit Result | ASTM D7566 Limit |
|---|---|---|---|
| Thermal Oxidative Stability (D3241) | |||
| Pressure Drop (mm Hg) | ≤ 10 | 7 | ≤ 25 |
| Tube Deposit Code | ≤ 1 | 1 | ≤ 3 |
| Flash Point (°C, D93) | ≥ 52 | 58 | ≥ 38 |
| Density @ 15°C (kg/m³, D4052) | 775 - 840 | 792 | 730 - 770 |
| Aromatics (% vol, D6379) | ≤ 26.5 | 22.1 | ≤ 26.5 |
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials for Biomass SAF Handling & Distribution Research
| Reagent/Material | Function in Research Context |
|---|---|
| Certified Hydroprocessed Esters & Fatty Acids (HEFA) | Reference standard for establishing dedicated line cleaning efficiency and commingling baselines. |
| Synthetic Iso-Paraffins (SIP) from Hydroprocessed Fermented Sugars | Used in batch sequencing studies as a high-purity, low-contaminant model fluid. |
| Tracers (e.g., Perfluorocarbon Tracers, PFTs) | Injected in nanogram quantities to track batch interfaces and detect cross-contamination with high sensitivity. |
| Pipeline Material Coupons (Carbon Steel, Stainless Steel 316L) | Used in static immersion experiments to study catalytic effects of wall materials on SAF stability. |
| Industrial-Grade Nitrogen (Oxygen-Free) | Essential for creating inert blankets in storage vessels and as a spacer fluid in batch sequencing simulations. |
Visualization: Experimental Workflow for Commingling Protocol Validation
Title: SAF Commingling Protocol Validation Workflow
Visualization: Dedicated Line Purging Logic Decision Tree
Title: Dedicated Line Purging Decision Logic
Specialized Filtration and Dehydration Systems for High-Purity Fuel Handling
Technical Support Center: Troubleshooting & FAQs
Thesis Context: This technical support center is developed as a component of a doctoral thesis on "Infrastructure adaptation for biomass SAF handling and distribution research." The guidance provided addresses the precise filtration and dehydration challenges encountered in research-scale, high-purity fuel handling for advanced biofuel and synthetic fuel development.
Troubleshooting Guides
Issue: Rapid Pressure Drop Increase Across Particulate Filter
- Observed Symptoms: System flow rate decreases unexpectedly. Pump noise may increase. Differential pressure gauge reading exceeds manufacturer's recommended maximum.
- Likely Causes & Solutions:
- Cause: Excessive solid contaminant load from biomass-derived SAF (e.g., catalyst fines, unconverted lignin residues).
- Solution: Pre-filter fuel with a higher micron-rated depth filter (e.g., 10 µm) upstream of the final 0.5 µm absolute filter. Increase sampling frequency for feedstock impurity analysis.
- Cause: Filter media incompatibility with fuel additives (e.g., certain antioxidants or metal deactivators).
- Solution: Consult filter manufacturer chemical compatibility charts. Switch to media rated for aggressive hydrocarbons (e.g., PTFE membrane, borosilicate glass microfiber).
- Protocol for Diagnosis: Isolate the filter housing. Measure inlet and outlet pressure. Calculate ∆P. If ∆P > 30 psi, replace filter cartridge. Retain the used cartridge for post-mortem analysis (e.g., SEM, EDX) to identify contaminant composition.
- Cause: Excessive solid contaminant load from biomass-derived SAF (e.g., catalyst fines, unconverted lignin residues).
Issue: Failure to Achieve Target Water Specification (<30 ppm)
- Observed Symptoms: Karl Fischer titration results consistently show water content >30 ppm despite system operation. Visible haze in fuel sample.
- Likely Causes & Solutions:
- Cause: Saturation of adsorbent dehydration media (e.g., 3Å molecular sieves).
- Solution: Implement a scheduled regeneration protocol based on throughput, not time. For research systems, regenerate sieves after processing every 5 liters of biomass-SAF. See Regeneration Protocol below.
- Cause: Air ingress into storage vessels or lines, introducing atmospheric moisture.
- Solution: Maintain a positive pressure of dry nitrogen or argon blanket on all storage tanks and reservoirs. Conduct a pressure decay test on the fuel handling loop.
- Diagnosis Protocol: Sample fuel immediately before and after the dehydration vessel. Perform inline water activity measurement if available, or use sealed syringe sampling for immediate Karl Fischer analysis to isolate the dehydration unit's performance.
- Cause: Saturation of adsorbent dehydration media (e.g., 3Å molecular sieves).
Issue: Fuel Blending Inconsistencies Post-Filtration
- Observed Symptoms: Variability in measured cetane number or other key performance indicators after blending and filtration.
- Likely Causes & Solutions:
- Cause: Selective adsorption of polar biofuel components (e.g., certain ester molecules) onto filter or dehydrator media.
- Solution: Perform a mass balance analysis of the blend pre- and post-filtration using GC-MS. Consider using inert, surface-deactivated wetted materials (e.g., electropolished stainless steel, certain fluoropolymers).
- Protocol for Adsorption Test: Pass a known volume of blend through a new filter/dehydration cartridge. Collect all effluent. Precisely measure and compare the volume and composition of influent vs. effluent using calibrated methods.
- Cause: Selective adsorption of polar biofuel components (e.g., certain ester molecules) onto filter or dehydrator media.
Frequently Asked Questions (FAQs)
Q1: What is the recommended pore size for final particulate filtration of research-grade biomass SAF? A: For most downstream catalytic processes or fuel cell applications, a two-stage filtration approach is critical. A primary depth filter at 5-10 µm removes bulk particulates, followed by an absolute-rated membrane filter at 0.5 µm for final polishing. This protects sensitive equipment like catalyst beds or injectors from biomass-derived contaminants.
Q2: How often should molecular sieve desiccant beds be regenerated in a laboratory-scale fuel handling system? A: Regeneration frequency is throughput-dependent. For a typical 5-liter research system, regenerate after processing 3-5 liters of hygroscopic biomass-SAF. Monitor effluent water content with an inline sensor. The standard regeneration protocol involves: 1) Draining fuel, 2) Purging with dry nitrogen, 3) Heating to 250-300°C under vacuum or continuous nitrogen purge for 8-12 hours, 4) Cooling under dry purge.
Q3: Can standard petroleum-fuel filters be used for biofuels like SAF? A: Not without validation. Biomass-SAF often contains different chemical functionalities (esters, furanics) that can degrade standard elastomer seals (e.g., Buna-N) and be adsorbed by certain media. Always specify filters with wetted materials compatible with oxygenated hydrocarbons, such as Viton seals, PTFE membranes, and 316L stainless steel housings.
Q4: What is the most reliable method for measuring trace water in our high-purity fuel samples? A: For accuracy at the sub-50 ppm level, Karl Fischer (KF) titration is the benchmark. For process monitoring, tuneable diode laser absorption spectroscopy (TDLAS) offers real-time, inline data. See comparison table below.
Data Presentation
Table 1: Trace Water Measurement Technique Comparison
| Technique | Measurement Principle | Typical Range (ppm H₂O) | Accuracy (ppm) | Advantage for SAF Research |
|---|---|---|---|---|
| Karl Fischer Titration | Coulometric or Volumetric | 1 ppm - 5% | ± 0.5 ppm (coulometric) | Laboratory gold standard; high precision. |
| TDLAS (In-line) | Laser Absorption | 0-1000 ppm | ± 1-2 ppm | Real-time, non-contact; process control. |
| Capacitance Sensor | Dielectric Constant Change | 0-500 ppm | ± 5-10 ppm | Rugged, lower cost; suitable for tank monitoring. |
Table 2: Filter Media Chemical Compatibility for Common SAF Components
| Filter Media Material | HVO/HEFA | Alcohol-to-Jet | FT-SPK | Key Limitation |
|---|---|---|---|---|
| PTFE Membrane | Excellent | Excellent | Excellent | Lower temperature limit vs. sintered metal. |
| Borosilicate Glass | Excellent | Good | Excellent | Can be brittle; avoid high pH impurities. |
| Nylon 6,6 | Good | Poor (hydrolysis) | Good | Degrades with trace water in esters/alcohols. |
| Sintered 316L Steel | Excellent | Excellent | Excellent | Highest durability; can be cleaned/reused. |
Experimental Protocols
Protocol 1: Regeneration of 3Å Molecular Sieve Dehydration Beds
- Objective: Restore adsorption capacity of water-saturated molecular sieves.
- Materials: Oven capable of 300°C, vacuum pump, dry nitrogen source, temperature-controlled vessel.
- Methodology:
- Safely drain all fuel from the dehydration vessel.
- Purge the vessel with dry nitrogen at 5 SLM for 30 minutes to displace residual hydrocarbons.
- Transfer the sieve beads to a dedicated, clean tray and place in the oven.
- Heat to 280°C ± 20°C under a slight negative pressure or continuous dry nitrogen purge for 10 hours.
- Allow to cool to <50°C in the dry atmosphere.
- Immediately transfer reactivated sieves to the dehydration vessel and seal under dry nitrogen.
- Validation: Test regeneration efficacy by processing a control fuel sample with known water content (e.g., 100 ppm) and measuring effluent with KF titration.
Protocol 2: Filter Integrity Test (Bubble Point Test)
- Objective: Verify the integrity and installed correctness of a membrane filter cartridge.
- Materials: Filter housing, pressure gauge, nitrogen source, water or compatible wetting fluid.
- Methodology:
- Wet the filter membrane completely with the specified fluid (e.g., isopropyl alcohol for PTFE).
- Slowly apply pressurized nitrogen to the upstream side.
- Monitor the downstream for a continuous stream of bubbles.
- Record the pressure at which the first steady stream of bubbles appears (Bubble Point).
- Compare this value to the manufacturer's minimum Bubble Point specification for that filter grade. A value at or above spec confirms integrity.
Diagrams
Diagram 1: High-Purity SAF Handling Workflow
Diagram 2: Molecular Sieve Regeneration Cycle
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for High-Purity Fuel Handling Research
| Item | Function & Specification | Rationale for SAF Research |
|---|---|---|
| 0.5 µm PTFE Membrane Filter Cartridge | Final particulate removal. Absolute rated. PTFE wetted parts. | Chemically inert to oxygenated SAF components; provides sterile-grade filtration. |
| 3Å Molecular Sieve Beads (1.6-2.5 mm) | Adsorptive dehydration to <10 ppm water. Must be reactivatable. | Selective pore size for H₂O over fuel molecules; essential for hygroscopic biofuels. |
| Karl Fischer Coulometric Titrator | Trace water measurement. Capable of 1 ppm detection. | Gold-standard analytical method for validating system performance and feedstock specs. |
| Dry Nitrogen Purge System | Provides inert blanket gas. Must include pressure regulator and moisture trap. | Prevents oxidation and moisture ingress during all transfer and storage steps. |
| Electropolished 316L Stainless Steel Transfer Vessels | Sample holding and blending. Sealed with PTFE-faced seals. | Minimizes surface adsorption of polar fuel molecules, ensuring sample integrity. |
| In-line TDLAS Water Analyzer | Real-time, continuous water vapor measurement in gas lines. | Monitors dehydration bed breakthrough and storage tank headspace moisture. |
Technical Support Center
Troubleshooting Guides & FAQs
FAQ 1: Why does my stored Hydroprocessed Esters and Fatty Acids (HEFA-SPK) sample appear cloudy, and what are the implications?
- Answer: Cloudiness indicates the formation of wax crystals as the fuel temperature approaches its Cloud Point. This is the temperature at which the first solid crystals form. These crystals can clog filters and injectors in downstream handling systems. The cloud point for HEFA-SPK typically ranges from -10°C to -30°C but can vary based on feedstock and processing. Operating below this point without modification risks system failure.
FAQ 2: Our flow simulation for a distribution line shows intermittent pressure spikes at +5°C. What is the likely cause?
- Answer: This is likely due to operating near or below the fuel's Freeze Point (also called Pour Point). While the bulk fuel may still be liquid, crystalline structures increase viscosity dramatically, leading to laminar flow disruption and pressure spikes. The freeze point for biomass-based Synthetic Paraffinic Kerosene (SPK) is typically 5-10°C below its cloud point. Modifications to line insulation or active heating are required.
FAQ 3: What is the most critical cold soak filtration test for assessing winter operability of a new bio-blendstock?
- Answer: The ASTM D5972 - Standard Test Method for Freeze Point of Aviation Fuels is critical. For a comprehensive assessment, combine it with ASTM D5773 - Standard Test Method for Cloud Point of Petroleum Products and ASTM D5949 - Standard Test Method for Pour Point of Petroleum Products. These protocols simulate long-term cold exposure and measure the temperature at which flow ceases.
FAQ 4: How do we experimentally differentiate between cloud point and freeze point phenomena in a novel Fischer-Tropsch (FT) distillate?
- Answer: Use a controlled cooling bath with visual and viscosity monitoring. The cloud point is identified visually (ASTM D5773 or D2500). The freeze/pour point is determined by tilting the sample jar horizontally for 5 seconds; the temperature at which no movement is observed is recorded (ASTM D5949). A rheometer can provide quantitative viscosity data to correlate with these visual observations.
Table 1: Typical Cold Flow Properties of Select Biomass SAF Pathways
| SAF Pathway / Blendstock | Typical Cloud Point Range (°C) | Typical Freeze Point Range (°C) | Standard Test Method | Critical Handling Threshold |
|---|---|---|---|---|
| HEFA-SPK (UCO Feedstock) | -12 to -20 | -20 to -30 | ASTM D5773, D5972 | Storage > -10°C |
| FT-SPK (Lignocellulosic) | -15 to -25 | -25 to -40 | ASTM D5773, D5972 | Filtration > -15°C |
| ATJ-SPK (Isobutanol) | Below -40 | Below -60 | ASTM D5773, D5972 | Excellent inherent properties |
| 50/50 HEFA/Conventional Jet | -8 to -15 | -15 to -25 | ASTM D2500, D5949 | Blend-specific testing required |
Table 2: Efficacy of Cold Flow Improvers (CFIs) on Model HEFA Blends
| CFI Type (Concentration: 500 ppm) | Cloud Point Depression (°C) | Freeze Point Depression (°C) | Notes on Mechanism |
|---|---|---|---|
| Polyalkyl Methacrylate (PAMA) | 2 - 4 | 5 - 8 | Crystal modification & growth inhibition |
| Ethylene-Vinyl Acetate (EVA) Copolymer | 1 - 3 | 4 - 7 | Nucleation site interaction |
| Comb Polymer (e.g., PAMA-ST) | 3 - 6 | 8 - 12 | Combined adsorption & crystal distortion |
Experimental Protocols
Protocol: Determining Cloud and Freeze Points for Novel Bio-Blendstock (Adapted from ASTM D5773 & D5949)
- Sample Preparation: Filter 25 mL of test fuel through a 5µm filter to remove particulates. Transfer to a clean, dry test jar.
- Instrument Setup: Place sample jar in a controlled cooling bath (e.g., methanol cooled by dry ice or a programmable circulator). Insert a calibrated thermometer or PT100 probe.
- Cloud Point Procedure:
- Cool the sample at a rate of 1°C ± 0.5°C per minute.
- At each 1°C interval, remove the jar briefly and inspect visually against a specified background.
- Record the temperature at which a distinct haze or cloud is first observed at the bottom of the jar as the Cloud Point.
- Freeze/Pour Point Procedure:
- Continue cooling the sample from the cloud point at the same rate.
- At every 3°C interval, remove the jar and tilt horizontally.
- Record the temperature at which the sample shows no movement when the jar is held horizontal for 5 seconds as the Freeze Point.
- Replication: Perform in triplicate. Report the average.
Protocol: Evaluating Cold Flow Improver (CFI) Efficacy
- Blend Preparation: Prepare a 500 mL base blendstock of known cold flow properties. Add the target CFI (e.g., 500 ppm by mass) using a micropipette. Use a magnetic stirrer for 30 minutes at 40°C to ensure homogeneity. Prepare a control sample without CFI.
- Baseline Measurement: Determine the cloud and freeze points of the control sample using the protocol above.
- Treated Sample Measurement: Determine the cloud and freeze points of the CFI-treated sample.
- Calculation: Calculate the depression for each point: ΔT(°C) = T(control) - T(treated).
- Validation: Conduct a cold soak filtration test (ASTM D7214) at a target temperature (e.g., Cloud Point + 2°C) to assess real-world filterability improvement.
Visualizations
Title: SAF Production & Cold Property Evaluation Workflow
Title: CFI Action Mechanism on Wax Formation
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Cold Flow Property Research
| Item / Reagent | Function / Rationale |
|---|---|
| Polyalkyl Methacrylate (PAMA) Additives | Industry-standard Cold Flow Improver (CFI) used as a positive control to test efficacy and establish baseline depression levels for novel blendstocks. |
| Synthetic Paraffinic Kerosene (SPK) Reference Standards | Calibrated samples with known cloud/freeze points (e.g., from NIST or commercial suppliers) for validation of test apparatus and protocol accuracy. |
| ASTM Type II Cooling Bath Fluid (e.g., Methanol) | A low-viscosity, low-freeze point fluid for precise temperature control in cloud/freeze point apparatus per ASTM specifications. |
| Programmable Circulating Chiller | Provides stable, reproducible cooling rates (±0.5°C/min) essential for standardized testing and high-quality data generation. |
| Micron-Rated Syringe Filters (0.45 µm - 5 µm) | For removing particulate contaminants that can act as nucleation sites for wax crystals, ensuring consistent sample preparation. |
| Digital Viscometer with Peltier Temperature Control | Quantifies the impact of temperature on dynamic viscosity, providing supplementary data to visual cloud/freeze points. |
| Model Hydrocarbon Waxes (n-C16 to n-C24) | Used to create synthetic fuel mixtures for fundamental studies on crystal nucleation and growth kinetics under controlled conditions. |
Digital Twins and IoT for Monitoring Fuel Quality Through the Distribution Network
Technical Support Center
Troubleshooting Guide: Common IoT Sensor Network Issues
Issue 1: Erroneous Viscosity Readings from In-Line Sensors
- Symptoms: Reported viscosity values from IoT sensors are inconsistent with laboratory benchtop viscometer readings, showing sudden spikes or drops.
- Probable Cause: Sensor fouling due to particulate matter or high molecular weight components in biomass-derived Sustainable Aviation Fuel (SAF).
- Resolution:
- Initiate an automated cleaning cycle if the sensor supports it (e.g., solvent flush).
- Manually inspect and clean the sensor probe according to manufacturer specifications.
- Calibrate the sensor using certified reference fluids relevant to the SAF blend (e.g., neat HEFA or ATJ fuels).
- Check the Digital Twin model's data validation rules. Adjust the anomaly detection threshold for viscosity to account for known feedstock variability.
Issue 2: Digital Twin and Physical Asset Data Desynchronization
- Symptoms: The Digital Twin dashboard shows fuel quality parameters (e.g., acid number) that do not match the real-time data stream from the storage tank monitor.
- Probable Cause: Latency in the IoT data pipeline or a failure in the data ingestion service.
- Resolution:
- Verify the connectivity status of the specific IoT gateway (e.g., LPWAN, cellular modem) serving the asset.
- Check the message queue (e.g., MQTT broker, Azure IoT Hub) for backlogged messages.
- Restart the data ingestion microservice.
- Use the "Force Sync" function in the Digital Twin platform (e.g., Azure Digital Twins, AWS IoT TwinMaker) to reconcile the asset state.
Issue 3: False Positive Oxidation Stability Alerts
- Symptoms: The system triggers oxidation stability degradation alerts during periods of no pipeline activity, despite controlled storage conditions.
- Probable Cause: Temperature sensor drift or localized heating near a pipeline section influencing the predictive model.
- Resolution:
- Cross-reference temperature readings from adjacent sensors.
- Validate the alert against laboratory Rancimat test results from a physical sample.
- Retrain the machine learning model for oxidation prediction using a dataset that includes this specific "at-rest" scenario to reduce false positives.
Frequently Asked Questions (FAQs)
Q1: What is the recommended sampling frequency for IoT sensors to balance data fidelity and network load in a long-distance pipeline study? A1: For continuous monitoring of key fuel quality parameters (temperature, pressure, density), a 1-minute interval is sufficient. For more complex, power-intensive analyses (like spectroscopic composition estimation), a 5-10 minute interval is recommended. This can be adjusted based on network bandwidth and the criticality of the pipeline segment within your thesis research on distribution infrastructure.
Q2: How do I integrate new, experimental sensor data (e.g., for trace metal content) into the existing Digital Twin framework? A2:
- Ensure the new sensor can output data in a structured format (e.g., JSON) via an API or message protocol.
- Create a new "interface" in your Digital Twin model definition (e.g., using DTDL) for the trace metal property.
- Update the data ingestion workflow to map the incoming sensor data stream to this new twin property.
- The new data will now be available for visualization, analytics, and integration into predictive quality models.
Q3: Our research involves blending different batches of biomass SAF. How can the Digital Twin simulate the quality outcome of a blend before physical mixing? A3: Implement a "Blending Simulation" module within your Digital Twin environment. This module should:
- Ingest real-time quality data (ester content, acidity, viscosity) from the Digital Twins of the source storage tanks.
- Use pre-programmed mixing algorithms (e.g., linear blending rules for some properties, non-linear for others) based on your experimental data.
- Output a predicted quality profile for the proposed blend, which can be validated against a subsequent physical lab mix. This is core to testing infrastructure adaptability for variable feedstocks.
Experimental Data & Protocols
Table 1: IoT Sensor Performance in Monitoring SAF Blends
Data compiled from recent pilot-scale distribution network simulations.
| Sensor Parameter | Measurement Principle | Accuracy (vs. Lab Std) | Typical Sampling Interval | Susceptibility to Fouling (Biomass SAF) |
|---|---|---|---|---|
| Density | Coriolis / Vibrating U-tube | ±0.1 kg/m³ | 30 seconds | Low |
| Kinematic Viscosity | Microfluidic capillary | ±3% | 2 minutes | High |
| Water Content | Tunable diode laser abs. (TDLAS) | ±5 ppm | 1 minute | Medium |
| Acid Number (TAN) | Near-Infrared (NIR) Spectroscopy | ±0.05 mg KOH/g | 5 minutes | Medium (Requires frequent recalibration) |
Experimental Protocol: Validating Digital Twin Predictions for Oxidation Stability
Objective: To correlate Digital Twin-predicted oxidation stability (based on real-time IoT temperature & trace component data) with standardized laboratory analysis.
Methodology:
- Data Collection Phase: Over a 30-day storage period, continuously record fuel temperature and pre-processed NIR spectral data from the IoT-enabled storage tank.
- Digital Twin Prediction: The Digital Twin uses a trained model (e.g., Random Forest regression) to predict the Induction Period (IP) in hours daily, using the ingested IoT data.
- Physical Sampling & Validation: Every 72 hours, draw a 500ml sterile sample from the tank under an inert atmosphere (N2 blanket).
- Laboratory Analysis: Immediately analyze the sample using a standardized Rancimat instrument (EN 16091 or ASTM D7545). Record the measured IP.
- Correlation Analysis: Plot predicted IP (Digital Twin) vs. measured IP (Rancimat) and calculate the Root Mean Square Error (RMSE) and R² value to validate the model's accuracy for your specific SAF formulation.
Research Reagent Solutions & Essential Materials
| Item Name | Function/Application in Research | Key Consideration for Biomass SAF |
|---|---|---|
| Certified Reference SAF Blends (e.g., HEFA-SPK, FT-SPK) | Calibration of IoT sensors (NIR, density); baseline for quality experiments. | Must match the chemical composition (paraffinic, ester content) of the fuel under study. |
| Stabilizer & Antioxidant Additives (e.g., BHT, Tocopherols) | Used in controlled experiments to study oxidative degradation and sensor response. | Effectiveness can vary significantly between synthetic and bio-derived fuels. |
| Inert Gas Supply (N2 or Argon) | For creating inert headspace during sampling and storage experiments to prevent premature oxidation. | Critical for obtaining reliable baseline data on fuel degradation kinetics. |
| Standardized Test Kits (e.g., for Acid Number, Peroxide Value) | Provide ground-truth data to validate and recalibrate IoT sensor readings. | Must be validated for use with highly paraffinic, low-aromatic biomass SAF. |
| Microbial Growth Media Kits | To monitor and quantify microbial contamination in fuel/water interfaces in storage tanks. | Biofuels can have different microbial susceptibility profiles than conventional jet fuel. |
System Architecture & Workflow Diagrams
Solving Real-World Hurdles: Contamination, Degradation, and Efficiency Gaps
Preventing and Managing Microbial Contamination in Biomass Fuel Systems
Technical Support Center: Troubleshooting & FAQs
This support center is framed within a thesis on Infrastructure adaptation for biomass Sustainable Aviation Fuel (SAF) handling and distribution research. It provides targeted guidance for researchers and professionals encountering microbial issues in experimental biomass feedstocks and processing systems.
Frequently Asked Questions (FAQs)
Q1: What are the primary microbial contaminants in biomass fuel systems, and what risks do they pose? A: The primary contaminants are bacteria (e.g., Clostridium, Pseudomonas), fungi (molds and yeasts), and sulfate-reducing bacteria (SRBs). Risks include:
- Biodeterioration: Consumption of carbohydrates, lipids, and proteins, reducing feedstock calorific value.
- Acid Production: Microbial metabolism produces organic acids (e.g., lactic, acetic), lowering pH and corroding infrastructure.
- Biofilm Formation: Protects microbial communities, increasing resistance to biocides and causing flow blockages.
- Hydrogen Sulfide Production: By SRBs, leading to toxic atmospheres, corrosion, and catalyst poisoning in upgrading processes.
Q2: During lab-scale fermentation for bio-intermediate production, we observe a sudden pH drop and foul odor. What is the likely cause and immediate action? A: This indicates a contamination event, likely by acidogenic bacteria outcompeting the desired culture (e.g., ethanol-producing yeast). Immediate actions:
- Quarantine the bioreactor.
- Measure VFA (Volatile Fatty Acid) concentration via GC-MS.
- Take samples for Gram staining and microscopy to identify contaminant morphology.
- Do not proceed to downstream hydroprocessing; sterilize the batch and clean the system.
Q3: What are the best practices for preventing contamination in storage tanks for biomass-derived hydroprocessed ester and fatty acids (HEFA) intermediates? A: Implement a multi-barrier approach:
- Environmental Control: Maintain low water content (<0.1% w/w) and use inert gas (N₂) blanketing to reduce oxygen.
- Physical Cleaning: Schedule regular automated clean-in-place (CIP) cycles.
- Chemical Biocides: Use compatible, industry-approved biocides (see Table 1) in a "shock dosing" regimen, followed by monitoring of microbial adenosine triphosphate (ATP).
- Monitoring: Install real-time sensors for humidity and periodic test for microbial counts.
Q4: Which analytical methods are most effective for early detection of microbial contamination in solid biomass feedstocks like woody biomass or agricultural residues? A: A combination of culture-dependent and culture-independent methods is recommended.
| Method | Target | Time to Result | Detection Limit | Primary Use Case |
|---|---|---|---|---|
| ATP Bioluminescence | Cellular ATP | <5 minutes | ~100-1000 CFU/mL | Rapid field screening of slurry tanks |
| Polymerase Chain Reaction (qPCR) | Specific microbial DNA (16S rRNA genes) | 2-4 hours | ~10-100 gene copies | Speciation of SRBs in pipeline biofilms |
| Microbial Volatile Organic Compound (mVOC) Analysis (GC-IMS) | Microbial metabolite signatures | 10-30 minutes | ppm-ppb range | Early, non-detect of off-gassing from stored feedstocks |
| Standard Plate Count (R2A agar) | Viable, cultivable bacteria | 48-72 hours | 1 CFU/mL | Baseline assessment and strain isolation |
Experimental Protocols
Protocol 1: Assessment of Biocide Efficacy Against Pipeline Biofilm Simulants Objective: To evaluate the minimum biofilm eradication concentration (MBEC) of candidate biocides against a mixed-species biofilm relevant to biomass slurry pipelines.
- Biofilm Cultivation: Using a CDC biofilm reactor, cultivate a defined consortium (Pseudomonas aeruginosa, Bacillus subtilis, Desulfovibrio vulgaris) for 72 hours in a modified medium with 2% (w/v) biomass hydrolysate.
- Biocide Exposure: Transfer biofilm coupons to a 96-well MBEC assay plate. Serially dilute the biocide (e.g., glutaraldehyde, isothiazolinone) in fresh medium across rows. Expose biofilms for 4 hours at 25°C.
- Neutralization & Recovery: Rinse coupons twice with Dey-Engley neutralizing broth to stop biocide action. Place each coupon in a well containing fresh medium and sonicate for 5 minutes to disaggregate cells.
- Viability Quantification: Perform serial dilutions of the sonicate and plate on R2A agar. Count Colony Forming Units (CFU) after 48-hour incubation. Calculate MBEC as the lowest concentration reducing viability by ≥99.9% (3-log reduction) compared to untreated controls.
Protocol 2: Monitoring Microbial Community Shifts in Stored Biomass Feedstocks via 16S rRNA Gene Amplicon Sequencing Objective: To characterize temporal changes in the microbial population of herbaceous biomass (e.g., switchgrass) under different storage conditions.
- Sample Preparation: Triplicate samples of chipped biomass (500g each) are stored under: A) Ambient air, 30% moisture; B) N₂ blanket, 15% moisture. Sub-samples (10g) are collected weekly for 8 weeks.
- DNA Extraction: Use a commercial soil DNA extraction kit with a bead-beating step for rigorous cell lysis. Validate DNA quality via spectrophotometry (260/280 ratio ~1.8).
- Library Preparation: Amplify the V4 region of the 16S rRNA gene using universal primers (515F/806R). Attach Illumina sequencing adapters and indices via a dual-indexing PCR approach.
- Sequencing & Analysis: Pool libraries and sequence on an Illumina MiSeq platform (2x250 bp). Process raw reads using QIIME2 or Mothur pipelines for quality filtering, OTU clustering, taxonomy assignment (Silva database), and differential abundance testing (e.g., DESeq2).
Diagrams
The Scientist's Toolkit: Research Reagent Solutions
| Item | Function in Contamination Research | Example Product/Catalog # |
|---|---|---|
| Dey-Engley Neutralizing Broth | Inactivates residual biocides on sampled surfaces to allow accurate microbial enumeration. | Sigma-Aldrich, D3435 |
| ATP Bioluminescence Assay Kit | Provides reagents for rapid, on-site quantification of total microbial biomass via light emission. | Hygiena, SystemSURE Plus |
| Universal 16S rRNA Gene Primers (515F/806R) | Amplifies bacterial/archaeal DNA for community analysis via next-generation sequencing. | Illumina, 16S Metagenomic Sequencing Library Prep |
| CDC Biofilm Reactor | Standardized equipment for growing reproducible, high-density biofilms on material coupons. | BioSurface Technologies Corp., CBR 90-2 |
| R2A Agar | Low-nutrient medium for recovery of stressed microorganisms from water and fuel systems. | Millipore, 1.01666.0500 |
| Glutaraldehyde Solution (Biocide Simulant) | A broad-spectrum biocide used as a positive control in efficacy testing experiments. | Sigma-Aldrich, G6257 |
| AnaeroPack System | Creates an anaerobic environment for culturing strict anaerobes like SRBs from samples. | Thermo Fisher Scientific, AnaeroPack Rectangular Jar |
Optimizing Blending Ratios and Techniques for Consistent Fuel Performance.
Technical Support Center
Troubleshooting Guides & FAQs
Q1: Our blend of hydroprocessed esters and fatty acids (HEFA-SPK) with Fischer-Tropsch Synthetic Paraffinic Kerosene (FT-SPK) shows inconsistent viscosity after storage. What could be the cause and how can we diagnose it? A: Inconsistent viscosity often points to incomplete miscibility or phase separation under varying temperature conditions. This is a critical issue for infrastructure adaptation as it can affect pumpability and filter performance.
- Diagnosis Protocol:
- Replicate Storage Conditions: Place identical blend samples (e.g., 50/50 HEFA/FT) in controlled environments at 4°C, 25°C, and 40°C for 72 hours.
- Visual & Microscopic Inspection: Check for haze or droplets. Use optical microscopy at 100x magnification to detect micro-phase separation not visible to the eye.
- Kinematic Viscosity Test: Measure viscosity at 40°C (ASTM D445) for each sample. A spread >0.1 mm²/s between samples indicates instability.
- FTIR Analysis: Perform Fourier-transform infrared spectroscopy to check for unexpected oxidation products or compositional shifts in the separated phases.
Q2: When blending alcohol-to-jet (ATJ) fuel with conventional Jet A-1, we observe rapid test failure for electrical conductivity. How can this be mitigated? A: ATJ fuels are highly pure and lack natural conductivity, which is a safety hazard during handling. The issue is directly relevant to distribution infrastructure, requiring additive dosing.
- Mitigation Protocol:
- Additive Screening: Prepare blends of ATJ with Jet A-1 at target ratios (e.g., 30% ATJ). Into each, dose Stadis 450 or similar static dissipater additive at 1, 2, and 3 ppm.
- Conductivity Measurement: Measure conductivity (ASTM D2624) immediately after mixing and again after 24 hours of equilibration.
- Optimal Dosing: Select the lowest additive concentration that achieves a stable conductivity >50 pS/m but <600 pS/m.
Q3: How do we systematically determine the maximum blend ratio for a novel Catalytic Hydrothermolysis (CH) jet fuel to maintain acceptable seal swell compatibility? A: Excessive seal swell can cause leaks; insufficient swell can cause brittleness. Testing is mandatory for pipeline and storage infrastructure compatibility.
- Experimental Protocol for Seal Swell:
- Sample Preparation: Create a series of blends with conventional fuel (e.g., CH-SPK at 10%, 30%, 50%, 70%).
- Seal Immersion: Immerse standardized nitrile (NBR) and fluorocarbon (FKM) O-rings in each fuel blend and a pure reference fuel control. Use triplicate samples.
- Conditioning: Age samples at 40°C for 168 hours (1 week).
- Measurement: Remove, blot dry, and measure volume change per ASTM D471. Calculate % volume swell.
Quantitative Data Summary
Table 1: Seal Swell Compatibility of CH-SPK Blends (NBR O-rings, 40°C, 168 hr)
| CH-SPK Blend Ratio (%) | Avg. Volume Swell (%) | Std. Deviation (±) | Within Spec (5-20%) |
|---|---|---|---|
| 0 (Reference Jet A-1) | 12.5 | 0.8 | Yes |
| 10 | 11.8 | 0.7 | Yes |
| 30 | 10.1 | 1.2 | Yes |
| 50 | 8.3 | 0.9 | No (Under-swell) |
| 70 | 6.7 | 1.1 | No (Under-swell) |
Table 2: Electrical Conductivity of ATJ/Jet A-1 Blends with Additive
| ATJ Blend Ratio (%) | Stadis 450 (ppm) | Conductivity (pS/m) @ 24h |
|---|---|---|
| 30 | 0 | <5 |
| 30 | 1 | 45 |
| 30 | 2 | 210 |
| 30 | 3 | 520 |
| 50 | 2 | 180 |
| 50 | 3 | 490 |
Experimental Workflow for Blend Stability Assessment
Title: Fuel Blend Stability Assessment Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for SAF Blend Research
| Item | Function | Example/Specification |
|---|---|---|
| Static Dissipater Additive | Increases electrical conductivity of pure synthetic fuels to meet safety standards. | Stadis 450, at various ppm concentrations. |
| Certified Reference O-Rings | Standardized materials for elastomer compatibility (seal swell) testing. | Nitrile (NBR) & Fluorocarbon (FKM) per AMS 7276. |
| Synthetic Paraffinic Kerosene (SPK) Standards | High-purity baseline components for creating controlled blends. | FT-SPK (ASTM D7566 Annex A1), HEFA-SPK (Annex A2). |
| Conductivity Meter | Measures picoSiemens per meter (pS/m) for fuel electrostatic hazard assessment. | ASTM D2624 compliant, with temperature control. |
| Precision Blending Apparatus | Ensures accurate volumetric or gravimetric mixing of fuel components. | Laboratory-scale mixer with <0.1% volume accuracy. |
| Stability Test Chamber | Provides controlled temperature environments for accelerated aging studies. | Capable of -10°C to 60°C, ±0.5°C stability. |
Pathway for Infrastructure Readiness Assessment
Title: From SAF Blend to Infrastructure Requirements
Addressing Thermal Oxidation Stability in Long-Duration Storage Scenarios
Technical Support Center
Troubleshooting Guides & FAQs
Q1: During accelerated oxidation testing of my biomass SAF blend, I observe a rapid increase in peroxide value after only 50 hours at 90°C, deviating from the expected profile. What are the likely causes and corrective actions?
A: A rapid peroxide value increase indicates a high concentration of readily oxidizable compounds, likely residual olefins or conjugated dienes from incomplete hydroprocessing. Insufficient antioxidant treatment is another primary cause.
- Troubleshooting Steps:
- Verify Feedstock & Processing: Confirm the hydrotreated ester and fatty acid (HEFA) or Fischer-Tropsch (FT) intermediate met all saturation specifications. Re-analyze for olefin content via GC-MS.
- Check Antioxidant Integrity: Test the concentration of the added antioxidant (e.g., BHT, TBHQ) in the blend using HPLC. Degradation or uneven mixing can occur.
- Assess Contaminants: Test for catalytic metal contaminants (e.g., Cu, Fe) via ICP-MS. Even ppm levels can catalyze decomposition.
- Corrective Protocol: If the issue is antioxidant-related, prepare a fresh antioxidant master batch (e.g., 10,000 ppm TBHQ in neat SAF) and re-blend to achieve the target dosage (typically 50-300 ppm). Re-run the accelerated test (e.g., Rancimat or modified ASTM D7545).
Q2: My sediment formation analysis in stored SAF samples shows high particulate counts (>25 mg/L) after 6 months of simulated storage. The chemical nature of the sediment is unknown. How should I proceed?
A: High sediment formation is a critical failure mode for storage infrastructure. The sediment is likely oxidized high-molecular-weight polymers or salt crystals.
- Diagnostic Workflow:
- Isolation: Filter the fuel through a pre-weighed 0.45 μm membrane. Wash with heptane and dry.
- Characterization:
- FT-IR: Identify functional groups (e.g., carbonyls from acids/ketones, hydroxides from alcohols).
- GC-MS of Soluble Fraction: Dissolve a portion in dichloromethane to identify low-MW oxidized species.
- Elemental Analysis (CHONS): Determine if sediment contains sulfur or nitrogen from feedstock impurities.
- Root Cause Analysis: Correlate sediment composition with the original fuel's total acid number (TAN) and heteroatom content. High TAN often precedes soap formation.
Q3: When testing the efficacy of different antioxidant packages, the results from the Pressurized Differential Scanning Calorimetry (P-DSC) and the traditional Rancimat method are contradictory. Which method should be trusted for long-term storage modeling?
A: Discrepancies are common. P-DSC (ASTM D6186) measures oxidation onset temperature under high oxygen pressure, favoring volatile antioxidants. Rancimat (EN 14112) measures induction time at a constant temperature, better simulating storage.
- Decision Table:
Method Key Parameter Best For Limitation for Storage Modeling P-DSC Oxidation Onset Temperature (OOT) Screening antioxidants at high pressure/speed. Overestimates performance of low-boiling antioxidants that may volatilize in long-term storage. Rancimat Induction Time (IT) Simulating ambient-temperature storage stability. Can underestimate stability of antioxidants that perform better at higher temperatures. - Recommendation: For long-duration storage scenarios, prioritize the Rancimat induction time data. Use P-DSC for initial, rapid screening. For your thesis on infrastructure adaptation, the Rancimat data directly correlates with allowable storage duration in tanks.
Key Experimental Protocols
Protocol 1: Accelerated Storage Stability Test (Modified ASTM D4625)
- Objective: Simulate long-term storage oxidation and assess sediment formation.
- Materials: SAF sample (500 mL), borosilicate glass tubes with Teflon-lined caps, oxygen manifold, controlled-temperature oven (±0.5°C), 0.45 μm membrane filters, analytical balance.
- Procedure:
- Purge four sample tubes with purified nitrogen for 5 minutes.
- Fill each tube with 250 mL of filtered SAF. Seal tightly.
- Place tubes in an oven set at 43°C (for a ~4x acceleration factor) or 60°C (for more aggressive testing).
- Remove one tube at pre-set intervals (e.g., 0, 3, 6, 12 months simulated).
- Analyze for: Peroxide Value (ASTM D3703), Total Acid Number (ASTM D664), and Filterable Solids (ASTM D4870).
- Data Integration: Plot values vs. time to create degradation curves for infrastructure maintenance scheduling.
Protocol 2: Antioxidant Synergism Screening via Isothermal Thermogravimetric Analysis (ITGA)
- Objective: Efficiently evaluate primary antioxidant (e.g., hindered phenol) and secondary antioxidant (e.g., peroxide decomposer) combinations.
- Materials: TGA with auto-sampler, aluminum pans, nitrogen and air gas supply, antioxidant stock solutions.
- Procedure:
- Prepare SAF blends with single antioxidants and combinations at varying ratios (e.g., 100:0, 75:25, 50:50, 25:75, 0:100 of primary:secondary).
- Load 10 ± 0.1 mg of each sample into a TGA pan.
- Ramp temperature to 150°C under N₂ at 50°C/min.
- Hold isothermally at 150°C for 2 minutes, then switch gas to synthetic air.
- Monitor mass loss until complete oxidation. The time to 5% mass loss in air is the key metric.
- Calculate synergism factor: SF = (ITcombination) / [(ITprimary * fractionprimary) + (ITsecondary * fraction_secondary)]. SF > 1 indicates positive synergism.
Diagrams
Title: Radical Chain Oxidation & Antioxidant Inhibition
Title: Stability Assessment Workflow for Infrastructure Planning
The Scientist's Toolkit: Research Reagent Solutions
| Item | Function & Relevance to SAF Storage Stability |
|---|---|
| Butylated Hydroxytoluene (BHT) | Primary hindered phenol antioxidant. Donates H-atom to peroxyl radicals (ROO•), terminating chain propagation. Baseline for performance comparison. |
| Tocopherols (e.g., δ-Tocopherol) | Natural primary antioxidant. Examined for "green" additive packages to improve sustainability profile of biomass SAF. |
| Tris(3,5-di-tert-butyl-4-hydroxybenzyl)isocyanurate | High-molecular-weight, non-volatile phenolic antioxidant. Key for long-duration storage where volatility loss is a concern. |
| Bis(2-ethylhexyl) sebacate | Representative synthetic ester used as a stable calibrant and blending component in oxidation experiments. |
| Cerium Oxide Nanoparticles | Investigational pro-oxidant catalyst. Used in controlled studies to understand the impact of trace contaminant metals on degradation rates. |
| Deuterated n-Hexadecane (C16D34) | Internal standard for GC-MS analysis of oxidation volatile organic compounds (VOCs). Allows precise quantification of degradation products. |
| Rancimat Conductivity Cells | Disposable cells containing deionized water for trapping volatile acids (e.g., formic, acetic) during Rancimat testing. Critical for induction time measurement. |
| Filter Membranes (0.1-0.45 μm, PTFE) | For quantitative isolation of insoluble oxidation solids (sediment/gum) from stored fuel samples. Pre-weighed for gravimetric analysis. |
Technical Support Center: Troubleshooting & FAQs for SAF Hydrant System Research
FAQs for Researchers on Biomass SAF Hydrant Infrastructure
Q1: During a compatibility simulation, what are the primary degradation indicators for legacy hydrant system elastomers when exposed to biomass SAF components?
- A: Key indicators include swelling >15% volume change, hardness reduction >10 points Shore A, and tensile strength loss >25%. Monitor for specific trace compound leaching, such as esters or sulfides, from nitrile rubber O-rings or PTFE seals into the fuel stream, which can be analyzed via GC-MS. This is critical for assessing retrofitting feasibility.
Q2: Our flow modeling for a retrofitted system shows unexpected pressure drops. What are the most likely causes related to SAF properties?
- A: Biomass SAF often has different density and viscosity profiles than conventional Jet A. A mismatch between the assumed model fluid properties and the actual SAF blend being tested is the most common cause. Verify and input the exact temperature-dependent kinematic viscosity (ν) and density (ρ) of your specific SAF feedstock into your computational fluid dynamics (CFD) model. Secondly, check for assumed wall roughness; some SAF blends may have different lubricity, affecting friction factors.
Q3: When designing a new build hydrant system for dedicated SAF use, what are the top three material selection criteria beyond standard aviation fuel requirements?
- A: 1) Compatibility with a wide range of oxygenated compounds (e.g., aromatics-free SAF): Specify high-density polyethylene (HDPE) liner pipes or fluoropolymer coatings. 2) Resistance to microbial growth: Select materials that can integrate with or withstand regular biocide treatments common in biomass-derived fuels. 3) Low permeability to prevent moisture ingress: SAF can be more hygroscopic; consider bonded or welded barrier layers in pipework.
Q4: How do we establish a baseline corrosion rate for legacy airport hydrant piping to inform a retrofit decision?
- A: Implement a coupon testing protocol. Install pre-weighed, material-matched metal coupons (e.g., carbon steel, stainless steel) into a test loop or at key points in a decommissioned leg of the existing system. Circulate both conventional Jet A and your target biomass SAF blend under controlled temperature and simulated static/dynamic conditions for a minimum of 1000 hours. Measure mass loss and perform surface pitting analysis via profilometry.
Experimental Protocols for Infrastructure Adaptation Research
Protocol 1: Elastomer Compatibility & Degradation Testing
- Objective: To quantify the chemical compatibility of legacy hydrant system sealing materials with novel biomass SAF.
- Materials: Test coupons (NBR, FKM, PTFE), immersion chambers, temperature-controlled oven, digital calipers, durometer, tensile tester, GC-MS.
- Methodology:
- Prepare control (Jet A) and experimental (biomass SAF) fuel samples.
- Immerse pre-measured elastomer coupons in fuels at 40°C and 60°C to simulate ground and elevated temperatures.
- At intervals (168h, 672h, 2016h), remove coupons, blot dry, and measure:
- Volume change (via fluid displacement).
- Hardness change (Shore A scale).
- Tensile strength and elongation at break.
- Analyze fuel for leachates via GC-MS.
- Data Analysis: Compare percentage changes against ASTM D471 and D7216 standards. Failure thresholds are defined per system operator specifications.
Protocol 2: Hydraulic Performance Benchmarking
- Objective: To compare pressure drop and flow efficiency between a retrofitted legacy system design and a new build design optimized for SAF.
- Materials: CFD software (e.g., ANSYS Fluent), system CAD models, validated SAF property data (viscosity, density as f(T)).
- Methodology:
- Create two models: "Retrofit" (existing pipe geometry with updated internal roughness) and "New Build" (optimized pipe diameters, layout, and materials).
- Define boundary conditions: pump curve, tank head, target mass flow rate.
- Set fluid properties for conventional fuel and three distinct biomass SAF blends (e.g., HEFA, ATJ, FT-SPK).
- Run steady-state and transient simulations for multiple demand scenarios (single gate, peak all gates).
- Data Analysis: Extract system curve data, nodal pressures, and identify potential cavitation or low-flow zones. Compare capital (CAPEX) and operational energy (OPEX) costs.
Quantitative Data Summary
Table 1: Comparative Cost-Benefit Analysis Framework
| Metric | Retrofitting Legacy Hydrant System | New Build Hydrant System |
|---|---|---|
| Estimated Capital Cost (CAPEX) | 30-60% of new build cost. Highly variable based on condition. | Baseline (100%). Higher initial outlay. |
| Key Cost Drivers | System flushing, inline inspection, seal/gasket replacement, partial pipe lining, filtration upgrades. | Land acquisition, trenching, new piping/valves, control systems, full compliance with latest codes. |
| Operational Risk (OPEX) | Higher short-term risk of leaks/material incompatibility. Potential for unplanned downtime. | Lower long-term risk. Designed for SAF compatibility from outset. |
| System Optimization Potential | Low to Moderate. Constrained by existing layout. | High. Can be optimized for efficiency, future expansion, and dedicated SAF handling. |
| Project Timeline | Shorter (Can leverage existing rights-of-way). | Longer (Requires new permits, construction). |
| Best Suited For | Airports with robust existing infrastructure and lower initial SAF blend ratios. | New airports, major expansions, or hubs targeting >50% SAF blend in the near term. |
Research Reagent Solutions & Essential Materials
Table 2: Key Research Materials for SAF Hydrant Compatibility Testing
| Item / Reagent | Function in Experiment |
|---|---|
| Biomass SAF Blends (HEFA, FT, ATJ) | Test fluids representing real-world chemistry to assess material compatibility and hydraulic performance. |
| Certified Jet A-1 Reference Fuel | Control fluid for establishing baseline performance and degradation rates. |
| NBR, FKM, EPDM Elastomer Coupons | Standardized samples of common sealing materials to test for swelling, hardening, and leaching. |
| Carbon & Stainless Steel Coupons | For measuring corrosion rates, pitting, and general degradation in fuel-wetted environments. |
| Inline Optical Particle Counter | Monitors for particulate generation from material degradation or microbial growth in test loops. |
| GC-MS (Gas Chromatography-Mass Spectrometry) | Analyzes fuel composition pre- and post-exposure to identify trace compounds, additives, or leachates. |
| Hydraulic Test Loop (Bench-Scale) | Simulates flow, pressure, and temperature conditions of a full-scale hydrant system for controlled study. |
Visualizations
Diagram 1: Infrastructure Adaptation Decision Workflow
Diagram 2: SAF-Elastomer Interaction Pathways
Personnel Training and Safety Protocols for Handling Novel Fuel Components
Technical Support Center: Troubleshooting & FAQs
This center provides support for researchers conducting experiments related to novel biomass-derived Sustainable Aviation Fuel (SAF) components. The content is framed within the thesis: "Infrastructure Adaptation for Biomass SAF Handling and Distribution Research."
Frequently Asked Questions (FAQs)
Q1: During the oxidative stability testing of a novel terpene-based fuel blend, we observed rapid peroxide formation. What are the likely causes and mitigation steps? A1: Rapid peroxide formation indicates autoxidation. Likely causes include:
- Presence of natural antioxidants from biomass (e.g., tocopherols) in insufficient quantities or their degradation during feedstock processing.
- Trace metal contamination (Co, Mn, Fe) from reactor vessels or storage tanks acting as pro-oxidants.
- Excessive exposure to light and oxygen during sample handling.
- Mitigation: Implement strict sample handling under inert atmosphere (N₂), use metal chelators (e.g., EDTA), add synthetic antioxidants (e.g., BHT, TBHQ) at 50-200 ppm, and conduct storage in amber vials.
Q2: Our viscometry readings for a synthesized iso-paraffin are inconsistent with modeled predictions. How should we troubleshoot the measurement? A2: Inconsistencies often stem from sample preparation or instrument calibration.
- Step 1: Ensure the sample is fully homogeneous and free of micro-bubbles or suspended particulates. Filter through a 0.2 µm PTFE filter.
- Step 2: Verify the precise temperature control of the viscometer bath. A ±0.1°C deviation can cause significant error for some compounds.
- Step 3: Calibrate the instrument using NIST-traceable viscosity standards in the range of your expected measurement.
- Step 4: Confirm the shear rate. Some bio-blends exhibit non-Newtonian behavior at low temperatures.
Q3: We suspect microbial contamination in our hydroprocessed esters and fatty acids (HEFA) intermediate storage tank. What protocols confirm this and how do we remediate it? A3: Microbial growth (bacteria, fungi) is possible in blends with residual oxygen and water.
- Confirmation Protocol: Aseptically sample from the tank bottom (water phase). Use ATP bioluminescence swabs for immediate results, or plate on R2A agar and incubated at 25°C and 37°C for 72 hours.
- Remediation: Drain all free water. Circulate and heat the fuel to 70°C for 4 hours if possible. For long-term storage, consider a registered fuel biocide (e.g., Biobor JF) at manufacturer-specified concentrations, ensuring compatibility with materials.
Q4: When blending furanic compounds (e.g., 2-Methylfuran) with conventional Jet A-1, we observe haze formation. What does this indicate and how is it resolved? A4: Haze formation indicates a phase separation or solubility limit issue, often due to water absorption or polarity mismatch.
- Diagnosis: Measure water content via Karl Fischer titration. If >200 ppm, water is the likely cause. If water is low, the blend has exceeded the solubility "pinch point."
- Resolution: (1) Use molecular sieves (3Å) to dry the blend. (2) Re-formulate the blend ratio, potentially adding a compatibilizer or co-solvent (e.g., a mid-range alcohol). (3) Ensure blending is done at a temperature at least 15°C above the cloud point of the mixture.
Key Experimental Protocols
Protocol 1: Determining the Hydrolytic Stability of Novel Ester-Based Fuel Components Objective: Assess the susceptibility of bio-derived esters to hydrolysis in the presence of water, which can form corrosive acids.
- Reagent Preparation: Prepare a 70:30 (v/v) mixture of the test ester component and deionized water. Adjust pH of the water phase to 5.0 using acetic acid/sodium acetate buffer to simulate acidic conditions.
- Procedure: Place 100 mL of the mixture in a sealed pressure tube with PTFE liner. Agitate constantly in a heated shaker bath at 60°C for 48 hours.
- Analysis: After cooling, separate the organic phase. Analyze for:
- Acid Number (ASTM D974): Measure the increase in total acid number (TAN).
- FT-IR Spectroscopy: Scan for the appearance of carboxylic acid (O-H stretch ~3000-2500 cm⁻¹, C=O ~1710 cm⁻¹) and reduction of ester C=O (~1740 cm⁻¹).
- Acceptance Criterion: A TAN increase of >0.5 mg KOH/g indicates poor hydrolytic stability for fuel applications.
Protocol 2: Accelerated Thermal Oxidation Test for SAF Components (Micro-Reactor Method) Objective: Rapidly assess oxidation stability and deposit formation tendency.
- Apparatus Setup: Load 5 mL of filtered fuel sample into a 10 mL stainless steel micro-reactor equipped with a pressure transducer and a removable metal coupon (common pipeline alloy, e.g., 316L).
- Procedure: Purge the reactor with O₂ for 1 minute. Pressurize with O₂ to 100 psig initial pressure. Place the reactor in a fluidized sand bath preheated to 180°C.
- Data Collection: Monitor pressure drop over 180 minutes. A rapid pressure drop indicates high O₂ consumption. After test, cool, vent, and remove the coupon.
- Post-Analysis: (a) Weigh coupon to determine insoluble deposit mass. (b) Rinse the reactor interior with tetrahydrofuran (THF) and analyze the soluble gums by Gel Permeation Chromatography (GPC).
- Quantitative Output: Report as Pressure Drop (psi) and Deposit Weight (mg/cm²). See Table 1.
Data Presentation
Table 1: Accelerated Oxidation Results for Candidate Components
| Component | Initial O₂ Pressure (psig) | Pressure Drop after 180 min (psi) | Deposit Weight on 316L Coupon (mg/cm²) |
|---|---|---|---|
| Jet A-1 (Reference) | 100 | 12 | 0.05 |
| HEFA (100%) | 100 | 18 | 0.12 |
| 2-Methylfuran (30% Blend) | 100 | 45 | 0.85 |
| Farnesane (100%) | 100 | 10 | 0.03 |
| Limonane (100%) | 100 | 22 | 0.15 |
Table 2: Common Fuel Property Analysis Methods & Standards
| Property | Standard Test Method | Typical Target for Jet Fuel | Key Apparatus |
|---|---|---|---|
| Flash Point | ASTM D93 | >38°C | Pensky-Martens Closed Cup Tester |
| Freezing Point | ASTM D5972 / D7153 | <-40°C to -47°C | Automated Phase Transition Analyzer |
| Thermal Stability | ASTM D3241 (JFTOT) | ΔP < 25 mm Hg | Jet Fuel Thermal Oxidation Tester (JFTOT) |
| Aromatics Content | ASTM D6379 | <25% (v/v) | Gas Chromatography with MS detector |
The Scientist's Toolkit: Research Reagent Solutions
| Item / Reagent | Function in SAF Research |
|---|---|
| BHT (Butylated Hydroxytoluene) | Primary antioxidant, radical scavenger used in stability tests at 50-300 ppm. |
| 3Å Molecular Sieves | Desiccant for drying fuel samples and standards to <10 ppm water for accurate analysis. |
| NIST-Traceable Alkanes (C10-C16) | Calibration standards for GC, GCxGC, and distillation curve analysis. |
| Metal Chelator (e.g., EDTA Disodium Salt) | Used in diagnostic tests to identify metal-catalyzed degradation by sequestering trace metals. |
| PTFE Membrane Filters (0.2 µm) | For sterile filtration of samples to remove particulates before microbial or instrumental analysis. |
| Jet A-1 Reference Fuel | Neat petroleum-based baseline for all comparative property testing and blending studies. |
| Karl Fischer Reagent (Coulometric) | Hygroscopic reagent for precise determination of water content in fuel samples (ppm level). |
Visualizations
Title: Thermal Oxidation Test Workflow
Title: Fuel Stability Issue Diagnosis Tree
Benchmarking Success: Validating Biomass SAF Infrastructure Against Alternatives
Technical Support Center: Troubleshooting Guides & FAQs
Thesis Context: This support content is developed within the research framework of Infrastructure Adaptation for Biomass SAF Handling and Distribution. It aims to assist researchers in addressing practical experimental challenges encountered during comparative lifecycle assessments (LCA) of aviation fuel pathways.
Frequently Asked Questions (FAQs)
Q1: During the biomass feedstock pre-processing stage for SAF, we observe inconsistent slurry viscosity in our hydrolysis reactor, leading to variable sugar yields. What are the primary troubleshooting steps? A1: Inconsistent viscosity often stems from feedstock variability. First, verify and standardize the particle size distribution (PSD) of your milled biomass (e.g., 2mm sieve cut-off). Second, calibrate the moisture content sensor for the incoming feedstock; target a consistent 10-15% (w/w) moisture. Third, check the pre-heating temperature stability; a fluctuation beyond ±2°C from the set point (typically 150-180°C) can significantly alter rheology. Implement a real-time viscosity probe with feedback control to the enzyme/acid catalyst pump rate.
Q2: In the Gas-to-Liquids (GTL) and Power-to-Liquids (e-SAF) pathways, catalyst deactivation in the Fischer-Tropsch (F-T) reactor is occurring faster than literature suggests. What factors should we investigate? A2: Accelerated deactivation in F-T synthesis is commonly linked to: (1) Poisoning: Analyze your syngas (for CTL) or H₂/CO₂ (for e-SAF) feed for contaminants. Sulfur must be <10 ppb, and nitrogen compounds <1 ppm. Use online GC-MS at the inlet. (2) Sintering: Monitor reactor bed temperature hotspots using a multi-point thermocouple array. A runaway exceeding 30°C above the set point (e.g., 220°C for Co-based catalysts) indicates poor heat management. (3) Carbon Deposition: Perform a Temperature-Programmed Oxidation (TPO) on a spent catalyst sample to quantify coke formation, which can be exacerbated by a low H₂/CO ratio (<1.8).
Q3: When conducting Life Cycle Inventory (LCI) analysis, how do we accurately allocate infrastructure burdens for a biorefinery co-producing SAF and bio-chemicals?
A3: This is a critical system boundary issue. We recommend a hybrid allocation method. First, apply energy-based allocation (exergy content) for the initial split at the gasification/synthesis stage. Then, for downstream separation, use economic allocation based on the 5-year average market price of the co-products (e.g., SAF vs. succinic acid). Ensure consistency with the ISO 14044 standard. The openLCA software with the ecoinvent v3.8 database contains pre-modeled allocation procedures you can adapt.
Q4: Our techno-economic analysis (TEA) model shows high sensitivity to hydrogen cost for the e-SAF pathway. What are the current benchmark values and sources for green H₂? A4: As of recent analyses (2023-2024), the levelized cost of green hydrogen from PEM electrolysis is highly scale-dependent. See Table 2 for current benchmark data.
Data Presentation Tables
Table 1: Comparative Infrastructure Footprint Key Performance Indicators (KPIs)
| Metric (Unit) | Biomass SAF (Gasification + F-T) | e-SAF (PtL, H₂ from PEM) | CTL (Coal-to-Liquids) |
|---|---|---|---|
| Feedstock Logistics Intensity (MJ-tonne/km) | 0.85 - 1.2 | Negligible | 0.25 - 0.4 |
| Plant Capital Intensity (USD per annual GJ SAF) | 110,000 - 145,000 | 180,000 - 300,000 | 90,000 - 120,000 |
| Water Consumption (m³/GJ SAF) | 1.8 - 3.5 | 1.1 - 1.8 (for electrolysis) | 4.0 - 8.0 |
| Carbon Utilization Efficiency (% feedstock C to SAF) | 35 - 45% | 55 - 65% (CO₂ to fuel) | 25 - 35% |
Table 2: Green Hydrogen Cost Benchmarks for e-SAF Modeling (2024)
| Electrolyzer Type | Scale (MW) | Assumed Capex ($/kW) | LCOH ($/kg H₂) | Key Assumption (Capacity Factor) |
|---|---|---|---|---|
| PEM (Current) | 10 | 1,200 - 1,500 | 4.8 - 6.2 | 60%, $50/MWh renewable power |
| PEM (Near-term) | 100 | 800 - 1,000 | 3.0 - 4.0 | 70%, $40/MWh renewable power |
| Alkaline (Current) | 100 | 600 - 900 | 2.8 - 3.8 | 85%, $35/MWh renewable power |
Experimental Protocols
Protocol 1: Determining Biomass Feedstock Slurry Rheology for Pipeline Transport Simulation Objective: To characterize the non-Newtonian flow behavior of lignocellulosic biomass slurries for pipeline design. Methodology:
- Sample Preparation: Mill biomass (e.g., corn stover, switchgrass) to pass a 2mm screen. Adjust moisture to 15% w/w.
- Slurry Formation: Mix biomass with deionized water at solid loadings of 15%, 20%, 25%, and 30% (w/w) in a high-shear mixer for 10 minutes.
- Rheometry: Using a rotational rheometer with a vane geometry, perform a shear rate sweep from 1 s⁻¹ to 1000 s⁻¹ at 25°C.
- Data Modeling: Fit the shear stress (τ) vs. shear rate (γ̇) data to the Herschel-Bulkley model: τ = τ₀ + K * γ̇ⁿ. Report yield stress (τ₀), consistency index (K), and flow behavior index (n).
- Pipeline Pressure Drop Calculation: Use the modeled parameters in the Darcy-Weisbach equation modified for non-Newtonian flow to estimate pressure drop per km for various pipe diameters.
Protocol 2: Accelerated Aging Test for e-SAF Electrolyzer Catalyst (PEM) Stability Objective: To simulate long-term degradation of IrO₂ anode catalysts in PEM electrolyzers under intermittent operation relevant to renewable grids. Methodology:
- Electrode Fabrication: Prepare membrane electrode assemblies (MEAs) with a standard loading of 2.0 mg IrO₂/cm² on the anode.
- Accelerated Stress Test (AST): In a single-cell test fixture (80°C), apply a cyclic potential between 1.4 V and 1.8 V RHE (Reference Hydrogen Electrode) with a 3-second hold at each vertex for 10,000 cycles.
- In-situ Monitoring: Record cyclic voltammograms (CV) every 500 cycles to track electrochemical surface area (ECSA) loss.
- Post-mortem Analysis: Perform X-ray Photoelectron Spectroscopy (XPS) on aged catalysts to quantify surface oxidation state changes and catalyst dissolution via Inductively Coupled Plasma Mass Spectrometry (ICP-MS) of the effluent water.
Mandatory Visualizations
Diagram Title: Biomass SAF Production Process Workflow
Diagram Title: Core Infrastructure Components Comparison
The Scientist's Toolkit: Research Reagent Solutions
| Item / Reagent | Function in Biomass SAF Infrastructure Research |
|---|---|
| Lignocellulase Enzyme Cocktail | Hydrolyzes cellulose and hemicellulose in biomass pre-processing to create fermentable sugars or more pumpable slurries. |
| Co-Precipitated Co/Al₂O₃ Catalyst | Standard Fischer-Tropsch synthesis catalyst for testing syngas conversion performance and product selectivity. |
| Certified Syngas Mixture Calibration Standard | (50% H₂, 25% CO, 15% CO₂, 10% N₂) Used for GC calibration in gasification and F-T process monitoring. |
| ICP-MS Multi-Element Standard Solution | For quantifying trace metal contaminants (e.g., S, Cl, K) in bio-oils and syngas that cause catalyst poisoning. |
| High-Temperature Rheometer with Vane Geometry | Measures viscosity and yield stress of high-solid biomass slurries under simulated process conditions. |
| PEM Electrolyzer Test Cell (5 cm²) | Bench-scale platform for evaluating catalyst (IrO₂, Pt/C) durability and membrane performance under AST protocols. |
| Life Cycle Inventory (LCI) Database Access (e.g., ecoinvent) | Provides background data on material/energy inputs for infrastructure construction and operation. |
| Process Modeling Software (Aspen Plus/ChemCAD) | Simulates mass/energy balances and optimizes process integration for infrastructure scale-up. |
Technical Support Center: Infrastructure Adaptation for Biomass SAF Handling & Distribution Research
This support center provides troubleshooting and methodological guidance for researchers investigating infrastructure compatibility, material performance, and fluid dynamics related to Sustainable Aviation Fuel (SAF) derived from advanced biomass feedstocks.
Frequently Asked Questions (FAQs) & Troubleshooting
Q1: In our simulated pipeline elastomer compatibility tests, we observe unexpected polymer swelling and reduced tensile strength beyond literature values. What could be the cause? A: This is frequently due to trace oxygenated compounds (e.g., specific furans or phenolic molecules) present in certain biomass-derived SAF blends that are not prevalent in conventional HEFA-SAF. Troubleshooting Guide: 1) Run Gas Chromatography–Mass Spectrometry (GC-MS) on your test fuel to identify specific polar contaminants. 2) Cross-reference with the elastomer's chemical resistance charts for those specific compounds. 3) Repeat immersion tests using a purer hydrocarbon baseline (e.g., neat n-dodecane) to confirm material baseline performance.
Q2: Our cold flow property analysis of a new bio-blendstock shows filter plugging points higher than predicted from pure component modeling. How should we debug this? A: This indicates potential crystallization or precipitation of minor high-melting-point constituents. Protocol: 1) Perform a detailed hydrocarbon-type analysis (via GCxGC or similar) to identify trace long-chain n-paraffins or wax esters. 2) Implement a modified ASTM D7501 (Cooling Rate Variation) test to assess the impact of thermal history. 3) Evaluate the effectiveness of commercial cold flow improvers at 0.1-0.3% dosage; record crystal morphology changes via microscopy.
Q3: When scaling up our catalytic upgrading process from batch to continuous flow, we encounter rapid pressure drop increase across the fixed-bed reactor. What are the primary investigative steps? A: This points to coke formation or feed instability. Action Steps: 1) Perform Thermogravimetric Analysis (TGA) on the spent catalyst under air to quantify coke yield. 2) Analyze the feedstock for potential contaminants (metals, phosphorous) via ICP-OES that could poison catalysts and induce coking. 3) Review pre-treatment protocols for oxygen removal; even low levels of oxygenates can accelerate oligomerization on acid sites.
Q4: In analyzing airport hydrant system residue after SAF blending, our microbial culture tests show persistent biofilm. How do we determine if the SAF blend or existing infrastructure is the source? A: This requires a controlled bioreactor experiment. Methodology: 1) Set up triplicate bioreactors with: a) Conventional Jet A, b) 50/50 SAF/Jet A blend, c) Positive control (Jet A + water + microbial inoculum). 2) Monitor microbial growth via ATP bioluminescence over 14 days. 3) Use 16S rRNA sequencing on the biofilm from the test case to identify predominant species and compare with historical airport pipeline biodata.
Table 1: SAF Uptake & Infrastructure Investment at Major Hubs (2023-2024)
| Airport Hub | SAF Volume (Million Liters, 2024 Est.) | Blend Ratio Target | Key Infrastructure Adaptation | Estimated Adaptation Cost (USD) |
|---|---|---|---|---|
| Los Angeles (LAX) | 60 | 10% by 2030 | Dedicated hydrant line from storage to Central Utility Plant for co-processing; modified fuel farm filtration systems. | $15 - 20 million |
| Singapore (SIN) | 15 | 1-5% initial uptake | New dual-purpose storage tanks with enhanced monitoring; segregated truck-loading bay for neat SAF at third-party terminal. | $8 - 12 million |
| San Francisco (SFO) | 45 | 5% by 2025 | In-line blending system with real-time density/RI monitoring; pipeline compatibility audits for all in-field seals. | $10 - 15 million |
Table 2: Common Material Compatibility Test Results (Accelerated Immersion, 28 days, 50°C)
| Material | Test Fluid (Conventional Jet A) | Test Fluid (HEFA-SAF Blend) | Test Fluid (Alcohol-to-Jet SAF) | Key Performance Delta |
|---|---|---|---|---|
| Nitrile O-Ring (NBR) | Volume swell: +5% | Volume swell: +8% | Volume swell: +15% | Exceeds spec limit with certain aromatics-free blends. |
| Viton O-Ring (FKM) | Volume swell: +2% | Volume swell: +1% | Volume swell: +3% | Generally compatible. |
| Polyurethane Sealant | No cracking | No cracking | Surface softening | Plasticization risk with oxygenates. |
Experimental Protocols
Protocol 1: Elastomer Seal Compatibility via Immersion Testing Objective: To quantify volume, mass, and hardness changes of sealing materials upon exposure to novel SAF blends. Methodology:
- Sample Preparation: Prepare standardized O-rings or coupons (per ASTM D471). Record initial mass (M1), volume via water displacement (V1), and Shore A hardness (H1).
- Immersion: Immerse samples in sealed vessels with test fuel (SAF blend and control) at 50°C for 28 days. Use a 20:1 fuel-to-sample volume ratio.
- Interim Measurement: Extract samples at 7-day intervals, blot dry, and measure interim mass (M2).
- Final Analysis: At 28 days, measure final mass (M3), volume (V3), and hardness (H3). Calculate percentage change.
- Post-Exposure Analysis: Perform tensile strength testing (ASTM D412) and FTIR analysis on dried samples to assess chemical alteration.
Protocol 2: In-Line Blending Homogeneity Verification Objective: To ensure consistent blend ratio across the transfer process from storage to aircraft. Methodology:
- Setup: Simulate an in-line blending system with a static mixer. Use dyed fluid for one component for visual confirmation.
- Sampling: Collect multiple 100mL samples from the output stream at 1-minute intervals over a 30-minute operational period.
- Analysis: Analyze samples using Fourier Transform Infrared (FTIR) spectroscopy calibrated for specific functional groups (e.g., ester carbonyl for HEFA) or via gas chromatography.
- Data Processing: Calculate the blend ratio for each sample. Determine the mean, standard deviation, and ensure it is within +/- 0.5% of the target ratio.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for SAF Infrastructure Research
| Item | Function / Rationale |
|---|---|
| Certified Reference Materials (CRM) | Neat SAF components (e.g., n-paraffins, iso-paraffins, aromatics) for calibrating analytical equipment and establishing baselines. |
| Specialized Elastomer Coupons | NBR, FKM, EPDM, etc., machined to ASTM specifications for standardized material compatibility testing. |
| Cold Flow Improver Additives | Polymeric compounds (e.g., ethylene-vinyl acetate copolymers) to study their efficacy on novel SAF blend crystallization kinetics. |
| Synthetic Microbial Consortia | Defined mixtures of bacteria and fungi known to inhabit jet fuel systems, for controlled biocontamination studies. |
| On-Line FTIR Probe with Flow Cell | For real-time, in-process monitoring of blend ratios or detection of specific functional groups during compatibility loops. |
| High-Pressure Rheometer | To characterize viscosity and flow behavior of viscous bio-blendstocks under simulated pipeline conditions (low temperature, high shear). |
Visualizations
Diagram 1: SAF Infrastructure Compatibility Testing Workflow
Diagram 2: Key Molecular Pathways in Microbial Degradation of SAF
Technical Support Center: Troubleshooting for Biomass SAF Handling and Distribution Simulation Research
FAQs and Troubleshooting Guides
Q1: Our simulation model for biomass feedstock logistics is producing unrealistically low cost estimates. What are the primary cost drivers we should validate? A: The most common cause is an oversimplified cost function. Ensure your model incorporates the following validated cost drivers, derived from recent industry benchmarks (2024-2025):
- Feedstock Pre-processing: Drying, grinding, and torrefaction energy costs.
- Handling & Storage: Capital and operational costs for specialized silos with moisture and temperature control to prevent biomass degradation.
- Transportation: Variable costs based on feedstock density (bulk vs. pelletized), mode (truck vs. rail), and distance, incorporating regional tariff data.
- Infrastructure Adaptation: Retrofitting costs for existing petroleum-based infrastructure to handle corrosive intermediates in biomass-to-SAF pathways.
Q2: How do we accurately model disruption events (e.g., port closures, harvest failures) in a supply chain simulation for global SAF distribution? A: Implement a multi-modal, time-stepped simulation with stochastic disruption triggers. Use historical data to parameterize event frequency and duration. The key is to model ripple effects (cascading failures) and alternative routing logic. See the experimental protocol below for a standard methodology.
Q3: Our agent-based model (ABM) of supplier behavior is not converging. What calibration steps are required? A: Agent-based models for biomass procurement require careful calibration of agent decision rules. Follow this protocol:
- Parameterize Agents: Define agent types (farmers, aggregators, biorefinery buyers) with distinct objective functions (profit maximization, risk aversion).
- Input Historical Data: Use past price, yield, and contract data to establish baseline behavior.
- Validate with Partial Equilibrium: Check that aggregated ABM outputs approximate results from a traditional economic equilibrium model for a stable historical period.
- Introduce Stochasticity Gradually: Add weather variability and market shocks only after baseline calibration is stable.
Q4: What are the critical data sources for validating a biomass feedstock geographic information system (GIS) model? A: You must integrate multi-source data. Primary validation sources include:
- USDA NASS CropScape: For land use and yield data.
- DOE Bioenergy KDF: For facility locations and capacities.
- FHWA Freight Analysis Framework: For road and rail network attributes and traffic.
- NOAA Climate Data: For historical weather disruption patterns.
Experimental Protocols for Cited Studies
Protocol 1: Modeling Disruption Resilience in a Multi-Echelon SAF Supply Chain
Objective: To quantify the impact of nodal disruptions on system-wide cost and SAF delivery reliability. Methodology:
- Network Definition: Map the supply chain as a directed graph with nodes (farms, pre-processing hubs, biorefineries, distribution terminals) and edges (transport links).
- Baseline Simulation: Run a discrete-event simulation over a 365-day horizon with nominal parameters. Record total cost and service level (on-time, in-full delivery).
- Introduce Disruptions: For each critical node (N), run n simulations where node N is inactivated for a randomly sampled duration (7-30 days) during the horizon.
- Resilience Metrics Calculation:
- Cost Increase (%):
(Cost_disrupted - Cost_baseline) / Cost_baseline * 100 - Service Level Degradation:
ServiceLevel_baseline - ServiceLevel_disrupted - Time to Recovery: Simulated days for system to return to baseline performance post-disruption.
- Cost Increase (%):
- Sensitivity Analysis: Repeat steps 3-4 for varying levels of safety stock/inventory policy at hubs.
Protocol 2: Validating Transportation Mode Cost Functions
Objective: To empirically derive cost-per-ton-mile functions for truck and rail transport of biomass feedstocks. Methodology:
- Data Collection: Gather 24 months of actual shipment data from industry partners or published case studies, including: distance, weight, mode, fuel surcharges, and freight rate.
- Regression Analysis: Perform multivariate linear regression to establish a cost function.
- Dependent Variable: Total freight cost ($).
- Independent Variables: Distance (miles), Weight (tons), Fuel Price Index, Route Type (rural/highway/intermodal).
- Simulation Integration: Embed the validated cost function into the larger supply chain simulation, ensuring it triggers correctly based on shipment attributes.
Data Presentation
Table 1: Validated Cost Drivers for Biomass Feedstock Logistics (2024 Data)
| Cost Driver Category | Specific Component | Typical Cost Range (USD/ton) | Key Modeling Variable | Source |
|---|---|---|---|---|
| Pre-processing | Mechanical Drying | $12 - $25 | Moisture % reduction, energy cost | DOE BETO Peer Review 2024 |
| Size Reduction (Grinding) | $8 - $15 | Feedstock type, target particle size | Ibid. | |
| Storage | Capital Cost (Silo) | $5 - $10 / ton capacity | Volume, aeration requirements | Industry Benchmarking |
| Loss & Degradation | $3 - $20 | Storage duration, climate control | ACS Sustainable Chem. Eng. 2025 | |
| Transportation | Truck (≤100 mi) | $0.30 - $0.55 / ton-mi | Density, backhaul availability | FAF5 Database 2023 |
| Rail (≥250 mi) | $0.08 - $0.15 / ton-mi | Siding access, volume commitment | Ibid. | |
| Infrastructure | Pipeline Retrofit | $1.2M - $3.5M / mile | Material compatibility, diameter | NREL Technical Report 2024 |
Table 2: Resilience Simulation Output for Key Supply Chain Nodes
| Disrupted Node Type | Avg. Cost Increase (%) | Avg. Service Level Drop (ppt) | Max Time to Recovery (Days) | Recommended Mitigation Strategy |
|---|---|---|---|---|
| Primary Pre-process Hub | 18.5% | 22.3 | 45 | Network redundancy; mobile pre-processing units |
| Major Biorefinery | 9.7% | 15.1 | 60 (plant restart) | Multi-sourcing agreements; regional inventory buffer |
| Key Rail Intermodal Yard | 5.2% | 8.7 | 28 | Pre-negotiated truck fleet contracts; alternate routes |
Diagrams
The Scientist's Toolkit: Research Reagent Solutions
| Item / Solution | Function in Simulation Research | Key Consideration for Biomass SAF |
|---|---|---|
| AnyLogic / Simio / FlexSim | Discrete-event and agent-based simulation platforms. | Ability to model complex logistics, queues, and resource constraints specific to bulk solids handling. |
| Python (Pyomo, SimPy) | Open-source optimization and simulation libraries. | Customizability for novel biomass degradation functions or unique policy rules. |
| GIS Software (ArcGIS, QGIS) | Spatial analysis and network analysis. | Critical for modeling feedstock catchment areas and real-world transportation networks. |
| Life Cycle Inventory (LCI) Database | Provides emission and energy use factors for unit processes. | Ensures environmental impact modeling (e.g., CI score) is coupled with cost/resilience analysis. |
| Stochastic Disruption Dataset | Historical data on port closures, weather events, demand shocks. | Must be tailored to agricultural and energy sectors for realistic perturbation modeling. |
| High-Performance Computing (HPC) Cluster | Executes thousands of simulation replications for robustness. | Essential for Monte Carlo analysis and comprehensive sensitivity testing across parameters. |
Technical Support Center
Frequently Asked Questions (FAQs)
Q1: Our analysis of a blended SAF sample shows a failed test for Thermal Oxidation Stability per ASTM D3241. The filter pressure drop is too high. What could be the cause?
- A: This is indicative of excessive particulate formation or insoluble gum precursors. In the context of biomass-derived SAF, this is often traced to trace oxygenates or polar compounds from the hydroprocessed esters and fatty acids (HEFA) or alcohol-to-jet (ATJ) pathways that are not fully removed. Contamination from storage tank sealants or hoses incompatible with aromatic-free fuels is also a common culprit. First, verify that the neat SAF (pre-blend) meets all specs. Then, audit the blending and transfer infrastructure for material compatibility.
Q2: We observe unexpected haze formation in a 50/50 SAF-conventional jet fuel blend stored in our simulated pipeline conditioning unit. What should we investigate?
- A: Haze typically indicates the presence of undissolved water or micelle formation from surfactants. For biomass SAF, this can be caused by residual esters, glycerides, or metals from catalysts that act as emulsifiers. Follow the protocol below to isolate the cause.
Q3: During a simulated custody transfer, our corrosion probe in the test loop shows higher than acceptable corrosion rates. The fuel meets acidity specs. What is the source?
- A: While organic acidity (ASTM D3242) may be within spec, biomass SAF can contain specific corrosive compounds not fully captured by standard tests, such as short-chain organic acids or sulfur species from feedstock variability. Additionally, dissolved oxygen ingress in the distribution system can exacerbate microbial growth (microbiologically influenced corrosion). Test for microbial content (ATP testing) and review dissolved oxygen controls.
Troubleshooting Guides
Issue: High Particulate Matter in Finished Fuel Blend (ASTM D2276 / D5452)
| Step | Action | Measurement Tool | Acceptable Threshold | Potential Root Cause (SAF-specific) |
|---|---|---|---|---|
| 1 | Isolate sample points (Pre-blend SAF, Post-blend, Post-filter). | Automated Particle Counter | < 6 mg/L (per D3241) | Determine if issue originates from SAF or blending. |
| 2 | Perform GC-MS scan for polar compounds. | Gas Chromatograph-Mass Spectrometer | Identify peaks not in conventional fuel. | Trace oxygenates (e.g., esters, aldehydes) acting as particle precursors. |
| 3 | Conduct a 4-week accelerated stability test (ASTM D4625). | Controlled Bath at 43°C | Visual & Particulate check at 2 & 4 weeks. | Inherent instability of SAF component reacting with trace metals (Cu, Zn) from infrastructure. |
| 4 | Material Audit: Swab test internals of blend vessel, hoses, pumps. | ICP-MS for Metal Analysis | Compare metal profile to new components. | Leaching of incompatible elastomers or galvanic corrosion from new material pairings. |
Issue: Phase Separation or Haze in Blended Fuel
| Step | Action | Measurement Tool | Acceptable Threshold | Protocol Reference |
|---|---|---|---|---|
| 1 | Perform a "Water Separation Index" test (Modified ASTM D3948). | WSIM apparatus | Rating ≥ 85 | Checks for surfactants. Low score indicates SAF-derived surfactants. |
| 2 | Test for dissolved water via Karl Fischer Coulometry (ASTM D6304). | Karl Fischer Titrator | ≤ 75 ppm | High dissolved water can precipitate with temperature swings. |
| 3 | Perform a freeze-thaw cycle (-40°C to 25°C) on the hazy sample. | Environmental Chamber | Visual clarity after 3 cycles | If haze clears, likely micro-droplets of water. If persistent, likely chemical incompatibility. |
Experimental Protocol: Assessing Material Compatibility of Elastomers with Neat SAF
Objective: To evaluate the volumetric swell and extractable content of common infrastructure elastomers (e.g., nitrile rubber, fluorocarbon) after exposure to 100% biomass-derived SAF.
Methodology:
- Sample Preparation: Cut elastomer coupons to 25mm x 50mm x 2mm. Weigh (W1) and measure volume (V1) via fluid displacement.
- Exposure: Immerse coupons in neat SAF (e.g., HEFA-SPK) in sealed vessels with zero headspace. Maintain at 40°C for 28 days.
- Post-Exposure Analysis:
- Swelling: Remove coupon, blot dry, and re-measure volume (V2). Calculate % volume swell = [(V2 - V1)/V1] * 100.
- Mass Change: Weigh dried coupon (W2). Calculate % mass change.
- Fuel Analysis: Analyze the exposed SAF via FTIR for signs of polymer degradation products (e.g., new carbonyl peaks).
- Control: Repeat with conventional Jet A-1 for baseline comparison.
The Scientist's Toolkit: Research Reagent & Material Solutions
| Item | Function / Rationale |
|---|---|
| Automated Particle Counter (per ASTM D7619) | Quantifies and sizes particles (≥4µm, ≥6µm, ≥14µm, ≥21µm, ≥25µm, ≥50µm, ≥100µm) to pinpoint filtration failure points. |
| Inductively Coupled Plasma Mass Spectrometry (ICP-MS) | Detects trace metal contamination (Fe, Cu, Zn, Na, K) at ppb levels, critical for identifying catalytic residues or infrastructure wear. |
| Solid Phase Extraction (SPE) Cartridges (Polar Modifiers) | Isolates polar oxygenates from SAF blends for subsequent GC-MS analysis to identify instability precursors. |
| Corrosion Coupon Rack (Carbon Steel, Aluminum, Silver) | Installed in a flow loop to measure long-term, cumulative corrosion caused by SAF blends under simulated pipeline conditions. |
| Water Separation Index Modified (WSIM) Kit | Quantifies the presence of surfactants which can stabilize water emulsions, a common issue with certain lipid-based SAF pathways. |
Diagram: Biomass SAF Infrastructure Compatibility Testing Workflow
Diagram: Fuel Stability Degradation Pathway Analysis
Welcome to the Technical Support Center for Infrastructure Adaptation Research in Biomass SAF Handling and Distribution. This resource provides troubleshooting guidance for common experimental and regulatory challenges.
FAQs & Troubleshooting Guides
Q1: Our lab is scaling up a novel biomass-derived SAF blendstock. What are the immediate regulatory reporting thresholds we must consider for volatile organic compounds (VOCs) during transfer operations? A: The primary trigger is the U.S. EPA's Risk Management Plan (RMP) rule under 40 CFR Part 68. For many common SAF precursors (e.g., ethanol, acetic acid, certain alcohols), the threshold quantity for reporting is 10,000 lbs on-site. Immediate steps are:
- Troubleshooting: If your process vessel exceeds this threshold, you must develop an RMP within one year. Failure triggers significant penalties.
- Protocol: Perform a real-time mass inventory calculation.
- Calculate the total weight (lbs) of each regulated substance in your largest process vessel + connected transfer lines.
- Use the formula:
Mass (lbs) = Volume (gal) × Density (lbs/gal). Density must be measured at process temperature. - If >10,000 lbs, initiate RMP procedures (Process Safety Information, Hazard Review, etc.).
Q2: We encountered an insurance premium surge after modifying our pilot-scale hydroprocessing unit to handle bio-oils with high acid content. What risk factors likely drove this? A: Insurers assess adaptation risks rigorously. Key factors are summarized below:
| Risk Factor | Typical Data Point | Impact on Insurance & Compliance |
|---|---|---|
| Material Compatibility | Corrosion rate > 0.5 mm/year for new bio-feedstock vs. < 0.1 mm for conventional feed. | Increases loss of containment risk; may violate equipment certification (ASME/API), voiding policy. |
| Process Safety Management (PSM) Update Lag | PHA revalidation delayed > 6 months post-modification. | Deemed a "Material Change" not managed; leads to coverage exclusions or premium increase of 50-200%. |
| Waste Stream Characterization | New aqueous phase by-product with COD > 100,000 mg/L. | Misclassification as "non-hazardous" can lead to RCRA violations and pollution liability claims. |
Q3: How do we formally document "infrastructure adaptation" for both a research grant auditor and an insurance underwriter? A: A unified Adaptation Impact Protocol is required.
Experimental Protocol: Adaptation Impact Documentation
- Objective: To systematically document the safety and compliance status of infrastructure modified for novel biomass SAF feedstocks.
- Materials: See "Research Reagent Solutions" below.
- Methodology:
- Baseline Capture: Document all original equipment specs (materials of construction, pressure ratings, relief valve set points).
- Change Specification: Record exact modifications (e.g., "Replaced section L34-T with Hastelloy C-276").
- Hazard Re-identification: Conduct a focused Process Hazard Analysis (PHA) only on the modified unit. Use a "What-If" checklist for new feedstock properties (corrosivity, oxygenates, polymerization potential).
- Compliance Gap Analysis: Map new process parameters against original operating permits (Air, NPDES) to identify reporting triggers.
- Generate Unified Dossier: Compile steps 1-4 into a single document with clear headers for insurer and auditor review.
Research Reagent Solutions
| Item | Function in Adaptation Research |
|---|---|
| Hastelloy C-276 Test Coupons | Immersion testing for corrosion rate quantification in novel, acidic bio-oil phases. |
| Miniature Pressure Relief Valve (PRV) Tester | Bench-top verification of PRV set points post-modification to ensure original design safety integrity is maintained. |
| Portable FTIR Gas Analyzer | Real-time monitoring of VOC emissions during transfer operations to determine reporting thresholds (LEL, TWA). |
| Wastewater Characterization Kit (COD, pH, TOC) | For classifying new aqueous process by-products to ensure correct hazardous waste handling and avoid liability. |
Visualization: Adaptation Compliance Workflow
Visualization: Risk Factor Interrelationship
Conclusion
The successful integration of biomass SAF into the aviation sector is fundamentally an infrastructure challenge, extending far beyond production alone. As explored, adaptation requires a deep understanding of fuel properties, innovative methodological approaches to storage and distribution, proactive troubleshooting of contamination and cold-flow issues, and rigorous validation against incumbent systems. For researchers and developers, this underscores a multidisciplinary frontier where materials science, logistics engineering, and data analytics converge. The future direction points towards hybrid infrastructure capable of handling diverse, drop-in sustainable fuels, necessitating continued R&D into compatible materials, smart monitoring systems, and standardized global protocols. The scaling of biomass SAF is not merely a fuel substitution but a systemic re-engineering of aviation's energy logistics, with profound implications for achieving net-zero targets in the decades ahead.